
16/01/2026
Turning lithium into battery-grade materials
Producing battery-grade lithium compounds is one of the final steps in the LiCORNE value chain. Partners across Europe have been refining electrochemical and crystallisation processes to recover lithium as high-purity carbonate or hydroxide from diverse sources: brines, ores and recycled cathode materials.
Electrodialysis for lithium hydroxide production
Using the solutions derived from VITO’s upstream processes, SINTEF researchers have constructed and commissioned electrochemical cells for electrodialysis to convert lithium chloride (LiCl) and sulfate (Li2SO4) solutions into lithium hydroxide (LiOH). Tests achieved:
- An optimal setup to produce LiOH at a specific energy consumption of 20 kWh/kg LiOH.
- A mixed product of Li₂CO₃ and LiOH via evaporative crystallisation, reaching almost 90% purity.
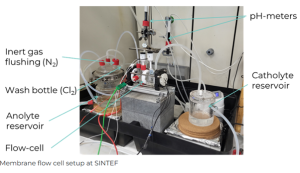
Membrane flow cell setup © SINTEF
Organic-based membrane electrolysis
TECNALIA, advancing the organic-based membrane electrolysis, scaled up to a 10 cm2 electrolysis cell to test three types of solutions –those produced by the liquid-liquid extraction processes from brines and from spodumene leachates, and the off-specification cathode leachates. Outcomes include:
| Off-specification cathode material | A four-chamber setup recovered lithium and oxalic acid with yields above 95%, while the carbonation process produced Li2CO3 of >99% purity. |
| Brines and spodumene | Li recovery is performed directly on the stripping dissolution obtained in the separation and purification steps, bypassing membrane-electrolysis. Carbonation delivered 88% purity for brines and 99% for spodumene. |
| PIMs [Polymer inclusion membranes] | Tests confirmed lithium migration is possible, but further research is needed to improve conductivity and ensure efficient transport. |
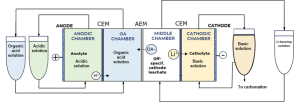
4-chamber flow cell diagram designed by TEC for lab experiments, © TECNALIA
Lithium purification and recovery via electrodialysis and electrolysis
The research group at Fraunhofer Institute for Chemical Technology ICT explored a simple, highly scalable method for Li2CO3 recovery using a combination of several methods like ion exchange (IE), reversed osmosis (RO), electrodialysis with bipolar membranes (EDBM), and Li2CO3-precipitation (see figure below). The goal was to recover high-purity lithium carbonate from Lithium-concentrated solutions provided by partners EnBW and KIT.
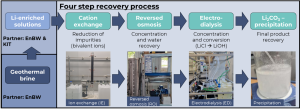
Setup for Li2CO3 recovery from Li-concentrated solutions starting with ion exchange, via reversed osmosis and electrodialysis | © Fraunhofer ICT
While ion exchange removed key impurities, the removal of Mn2+ ions (particular in EnBW samples) is still under investigation. Low contamination levels are crucial for electrodialysis and lifetime of EDBM. For KIT-sourced solutions, the process delivered Li₂CO₃ at 99.89% purity.
Selective chlorination and electrolysis of spodumene concentrate and waste cathode material
SINTEF researchers achieved selective chlorination of lithium from calcined spodumene concentrate and off-specification cathode waste, with yields exceeding 95%. Their selective chlorination converts insoluble oxides to soluble chlorides by electrolysis, thus extracting target elements: Li, Ni and Co. Experiments show:
- Li was selectively chlorinated from calcinated spodumene concentrate and off-specification cathode waste in NaCl-KCl-CaCl2 melts at yields >95%. Temperature and time play an important role in increasing yields, best results obtained above 700 °C. However, these melts are not feasible for Li electrowinning.
- Li was also chlorinated selectively from off-specification cathode waste at yields in LiCl-KCl mixture at temperatures ranging 470 – 850 °C, reaching yields of 59% for Li, and an upconcentrated residue of Co, Ni – a material that possibly could be validated further.

Chlorination setup at SINTEF, © SINTEF
Gas-diffusion electrocrystallisation
The Gas-Diffusion Electrocrystallisation (GDEx), VITO’s proprietary technology, achieved >95% lithium extraction from geothermal and continental brines, spodumene effluents and cathode leachates. Downstream synthesis produced Li₂CO₃ with:
- Energy consumption below 10 kWh/kg.
- >90% recovery across all tested matrices.
- Concentration increases of >1% from brines and >20% in solid eluates.
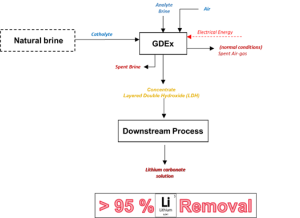
Schematic representation of the Gas-diffusion electrocrystallisation (GDEx) process, © VITO