16/01/2026
Lithium separation and purification
This content piece explores the advanced purification technologies developed by various LiCORNE partners to optimise lithium recovery from different sources, ranging from ore-derived streams and mineral leachates to geothermal brines and recycling. Researchers fine-tuned each method to deliver high yield and selectivity, addressing persistent challenges such as interference from competing cations and material stability over multiple cycles.
Porous lithium-ion sieves for enhanced packing density
VITO’s challenge was to develop 3D-structured inorganic Li-sieves with optimised composition, shape and porosity to adsorb lithium from complex aqueous leachates, with the ultimate aim to reach ≥98% Li+ selectivity, ≥95% Li+ yield, and <10% loss in performance over 10 cycles. Researchers developed a selective ion-exchange method using protonated titanium oxide [HTO] – a promising adsorbent for lithium recovery from aqueous leachates and brines due to its high selectivity and cycling stability.
VITO researchers recently achieved Li+ selectivity above 98 % and more than 90% yield in column tests. Currently, the system has the capacity to produce 1kg spheres per week. Although performance proved to be stable over multiple cycles, efficiency decreased by ~10% after five full cycles, each cycle lasting one full day, meaning that shorter operational cycles would extend the material’s usable lifetime.
VITO is applying for a patent on a methodology which avoids the dissolution of titanium (Ti) during the acidic regeneration treatment, ensuring no Ti is dissolved in any of the tested cycles.

HTO based spheres utilised for lithium extraction from spodumene leachates, © VITO
Ionic liquids
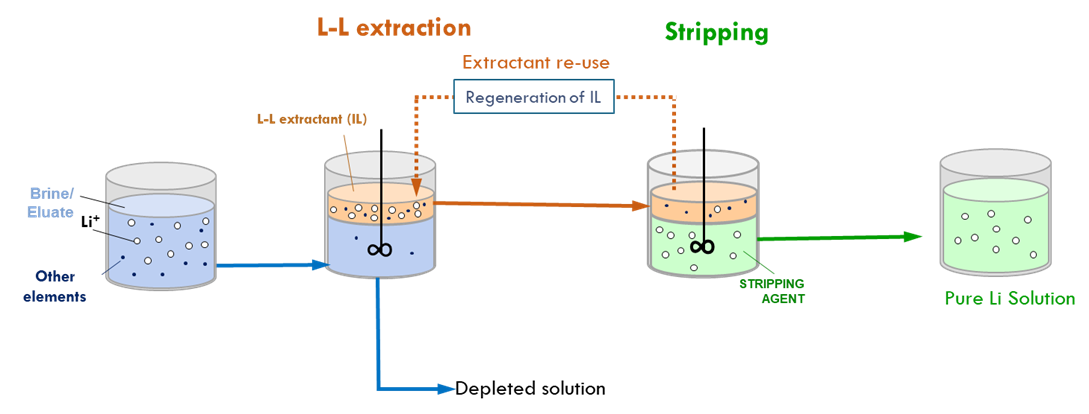
TECNALIA optimised ionic liquid-based extractants to recover lithium from geothermal and continental brines, as well as from leachates produced from mineral ores. These extractants offer an environmentally friendly alternative to conventional methods that use harsh acidic conditions. Based on lab experimental results, and using McCabe–Thiele simulations, these fine tunings were achieved for the global process including both steps the extraction and the stripping:
- 88% Li extraction yield for continental brine samples, and 86% for geothermal brine. Li+ selectivity reached approx. 99% for certain Li/cation combinations [e.g. 91% for K and 100% for Mg, with separation efficiencies up to 93%].
- 86% Li extraction yield for leachates from spodumene. Selectivity reached was around 99% for certain Li/cation combinations, e.g. 98% for Ca and 100% for Na, with a separation efficiency of 92%.
An additional benefit of this technology lies in its capacity to the reuse of the ionic liquids without compromising on the extraction performance.
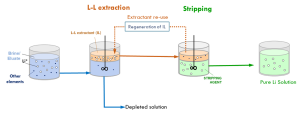
Liquid-liquid extraction & stripping process for the recovery of Li from brines and spodumene leachates © TECNALIA
Lithium extraction technology
The research and development department at EnBW has been advancing a Li+ extraction technology now at TRL4, designed to recover lithium from geothermal and continental brines through a sustainable process targeting yields of minimum 90%.
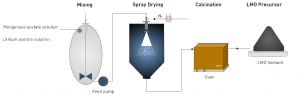
Li+ extraction technology © EnBW
The optimisation of their technology features a novel spray-drying process for Lithium Manganese Oxide [LMO] adsorbent. Doping the material with iron (Fe) or titanium (Ti), researchers improved capacity and chemical stability, which allowed researchers to recover up to 92% lithium from geothermal brines. Titanium doping proved particularly effective, significantly reducing manganese dissolution.
EnBW has recently filed a patent application for their LMO technology, which shows good potential for future implementation at industrial level for Li recovery.
Electrode-based Li adsorption and desorption from brines
KIT explored a dual-ion battery setup for direct lithium extraction from both continental and geothermal brines, as well as other Li-rich solutions recovered from EnBW’s process. Using Bi and FePO₄-based electrodes, latest results show:
- Li selectivity of 77% to 82% for geothermal brines, good separation from K and Mg, with Na and Ca being main contaminants.
- Li selectivity of 65-73% for continental brines (Na, K, Mg but also Sr as main contaminants).
Although researchers obtained higher recovery rates for continental brines [in the range of 27.7 to 39.9 mgLi/gLFP], selectivity remains a challenge.