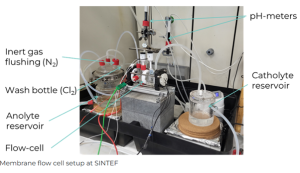
Membrane flow cell setup © SINTEF
Producing battery-grade lithium compounds is one of the final steps in the LiCORNE value chain. Partners across Europe have been refining electrochemical and crystallisation processes to recover lithium as high-purity carbonate or hydroxide from diverse sources: brines, ores and recycled cathode materials.
Using the solutions derived from VITO’s upstream processes, SINTEF researchers have constructed and commissioned electrochemical cells for electrodialysis to convert lithium chloride (LiCl) and sulfate (Li2SO4) solutions into lithium hydroxide (LiOH). Tests achieved:

Membrane flow cell setup © SINTEF
TECNALIA, advancing the organic-based membrane electrolysis, scaled up to a 10 cm2 electrolysis cell to test three types of solutions –those produced by the liquid-liquid extraction processes from brines and from spodumene leachates, and the off-specification cathode leachates. Outcomes include:
| Off-specification cathode material | A four-chamber setup recovered lithium and oxalic acid with yields above 95%, while the carbonation process produced Li2CO3 of >99% purity. |
| Brines and spodumene | Li recovery is performed directly on the stripping dissolution obtained in the separation and purification steps, bypassing membrane-electrolysis. Carbonation delivered 88% purity for brines and 99% for spodumene. |
| PIMs [Polymer inclusion membranes] | Tests confirmed lithium migration is possible, but further research is needed to improve conductivity and ensure efficient transport. |
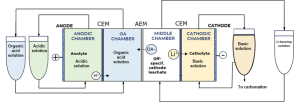
4-chamber flow cell diagram designed by TEC for lab experiments, © TECNALIA
The research group at Fraunhofer Institute for Chemical Technology ICT explored a simple, highly scalable method for Li2CO3 recovery using a combination of several methods like ion exchange (IE), reversed osmosis (RO), electrodialysis with bipolar membranes (EDBM), and Li2CO3-precipitation (see figure below). The goal was to recover high-purity lithium carbonate from Lithium-concentrated solutions provided by partners EnBW and KIT.
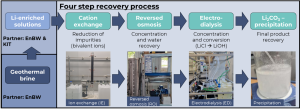
Setup for Li2CO3 recovery from Li-concentrated solutions starting with ion exchange, via reversed osmosis and electrodialysis | © Fraunhofer ICT
While ion exchange removed key impurities, the removal of Mn2+ ions (particular in EnBW samples) is still under investigation. Low contamination levels are crucial for electrodialysis and lifetime of EDBM. For KIT-sourced solutions, the process delivered Li₂CO₃ at 99.89% purity.
SINTEF researchers achieved selective chlorination of lithium from calcined spodumene concentrate and off-specification cathode waste, with yields exceeding 95%. Their selective chlorination converts insoluble oxides to soluble chlorides by electrolysis, thus extracting target elements: Li, Ni and Co. Experiments show:

Chlorination setup at SINTEF, © SINTEF
The Gas-Diffusion Electrocrystallisation (GDEx), VITO’s proprietary technology, achieved >95% lithium extraction from geothermal and continental brines, spodumene effluents and cathode leachates. Downstream synthesis produced Li₂CO₃ with:
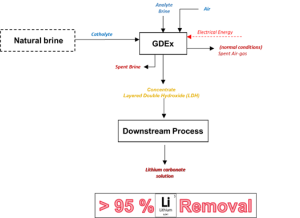
Schematic representation of the Gas-diffusion electrocrystallisation (GDEx) process, © VITO
The most recent political events and regulatory context have highlighted on several occasions a growing demand for lithium (Li), primarily fuelled by the increasing use of lithium-ion batteries (LIBs). The concentration of the largest mining sites for Li outside of Europe leads to a strong dependence on third countries, which can pose economic and strategic challenges for the EU. To mitigate the resources scarcity, the EU has implemented regulations promoting sustainable battery practices, and is actively exploring diversified domestic resources of Li, such as the geothermal brines located in the Rhine Graben region.
Researchers at Fraunhofer ICT recently attended the German Geothermal Conference in Essen (Germany), where they submitted a scientific poster that portrays the characteristics of the Li resources identified in the geothermal brines in the Rhine Graben region and the challenges raised by the Li extraction. Due to the high concentration of salt load, the selective separation of Li and sodium (Na) remains the primary challenge to solve. Within the LiCORNE project, researchers are testing a manganese-based (Mn-based) adsorption setup to provide Li enriched solutions from geothermal brines. Despite recent developments, impurities persist, and additional separation steps are required.
During the German Geothermal Conference, the research team at Fraunhofer ICT presented the free-flow electrophoresis (FFE) used for the selective separation of Li and Na ions. This separation relies on the ions’ migration velocity in an electrified field, being significantly influenced by their charge, size and hydrate shell.
The main advantage of the FFE lies in its capacity to prevent mixing of individual streams with the background eluent, allowing separate collection of the individual streams at the end of the chamber. Additional benefits of the FFE:
Testing the method with different parameters, various concentrations of the sample solution and altering the eluent solution, researchers reported a complete separation of Li and Na ions by FFE, with over 80% separation efficiency. Future efforts will focus on testing the actual desorption solutions, optimizing throughput, scaling up, and reducing costs.
Download the original poster, available in German, here