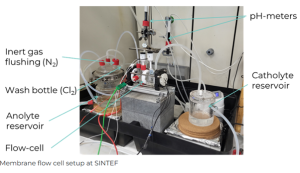
Membrane flow cell setup © SINTEF
Producing battery-grade lithium compounds is one of the final steps in the LiCORNE value chain. Partners across Europe have been refining electrochemical and crystallisation processes to recover lithium as high-purity carbonate or hydroxide from diverse sources: brines, ores and recycled cathode materials.
Using the solutions derived from VITO’s upstream processes, SINTEF researchers have constructed and commissioned electrochemical cells for electrodialysis to convert lithium chloride (LiCl) and sulfate (Li2SO4) solutions into lithium hydroxide (LiOH). Tests achieved:

Membrane flow cell setup © SINTEF
TECNALIA, advancing the organic-based membrane electrolysis, scaled up to a 10 cm2 electrolysis cell to test three types of solutions –those produced by the liquid-liquid extraction processes from brines and from spodumene leachates, and the off-specification cathode leachates. Outcomes include:
| Off-specification cathode material | A four-chamber setup recovered lithium and oxalic acid with yields above 95%, while the carbonation process produced Li2CO3 of >99% purity. |
| Brines and spodumene | Li recovery is performed directly on the stripping dissolution obtained in the separation and purification steps, bypassing membrane-electrolysis. Carbonation delivered 88% purity for brines and 99% for spodumene. |
| PIMs [Polymer inclusion membranes] | Tests confirmed lithium migration is possible, but further research is needed to improve conductivity and ensure efficient transport. |
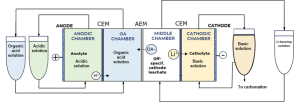
4-chamber flow cell diagram designed by TEC for lab experiments, © TECNALIA
The research group at Fraunhofer Institute for Chemical Technology ICT explored a simple, highly scalable method for Li2CO3 recovery using a combination of several methods like ion exchange (IE), reversed osmosis (RO), electrodialysis with bipolar membranes (EDBM), and Li2CO3-precipitation (see figure below). The goal was to recover high-purity lithium carbonate from Lithium-concentrated solutions provided by partners EnBW and KIT.
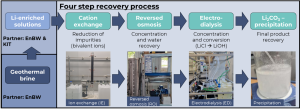
Setup for Li2CO3 recovery from Li-concentrated solutions starting with ion exchange, via reversed osmosis and electrodialysis | © Fraunhofer ICT
While ion exchange removed key impurities, the removal of Mn2+ ions (particular in EnBW samples) is still under investigation. Low contamination levels are crucial for electrodialysis and lifetime of EDBM. For KIT-sourced solutions, the process delivered Li₂CO₃ at 99.89% purity.
SINTEF researchers achieved selective chlorination of lithium from calcined spodumene concentrate and off-specification cathode waste, with yields exceeding 95%. Their selective chlorination converts insoluble oxides to soluble chlorides by electrolysis, thus extracting target elements: Li, Ni and Co. Experiments show:

Chlorination setup at SINTEF, © SINTEF
The Gas-Diffusion Electrocrystallisation (GDEx), VITO’s proprietary technology, achieved >95% lithium extraction from geothermal and continental brines, spodumene effluents and cathode leachates. Downstream synthesis produced Li₂CO₃ with:
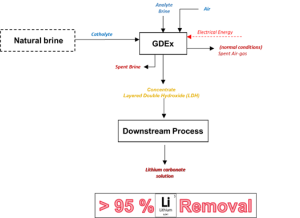
Schematic representation of the Gas-diffusion electrocrystallisation (GDEx) process, © VITO
The Horizon Europe project LiCORNE has completed an important milestone in its journey to establish a sustainable lithium supply chain in Europe. At the end of 30 months of research and technical development, the project consortium has selected three process flowsheets for upscaling. These routes represent the most promising routes for lithium recovery from European resources: ores, brines and off-specification battery cathode materials (waste).
Why this matters? Europe, from its position as an ambassador of the green transition, is expected to see a major increase in demand for lithium. Yet, its contribution to the lithium supply chain remains modest, despite holding an estimated 5 % of the global reserves. Most of this lithium is locked in hard-rock deposits, which are generally costly and environmentally challenging to extract. Domestic mining projects often face public resistance, while refining capacity remains limited.
Moreover, JRC’s studies indicate that despite a projected increase in EU’s battery cell production, the bloc remains import-reliant for battery-grade materials. Refined lithium inputs are expected to come increasingly from new EU mines, provided critical bottlenecks, such as domestic conversion and refining are removed. The Commission’s JRC additionally estimated that by 2040 recycled cobalt and nickel could meet up to 51 % and 42 % of EU demand, respectively.
LiCORNE, short for Lithium recovery and battery-grade materials production from European resources, is one of the numerous R&I initiatives launched to address this strategic vulnerability. The project aims to build Europe’s first integrated lithium supply chain. Its mission spans beyond simply optimising technological processes to recover lithium and battery-grade materials, aiming to provide solutions that are both efficient, scalable and sustainable.
After three years of research and technology optimisation, the LiCORNE consortium has selected the flowsheets that will be further upscaled during the project’s last 12 months. This selection followed a two-step assessment:
The final ranking identified the following three flowsheets as candidates for upscaling:
1. Spodumene route:
2. Continental brine route:
3. Off-specification cathode:
A feasibility study was performed for the three candidate flowsheets before moving into scale-up phase. The study confirmed their readiness for implementation in line with equipment requirements, scalability and the project’s remaining budget envelope.
In other research facilities, in different corners of Europe, other LiCORNE partners are reporting progress in producing battery-grade materials from various sources – brines, ores (spodumene for example) and off-specification cathode material.
Using the solutions derived from VITO-CAST team’s upstream processes, SINTEF researchers have constructed and commissioned electrochemical cells for electrodialysis of lithium chloride (LiCl) and lithium sulphate (Li2SO4) solutions. Researchers identified the optimal parameters to produce lithium hydroxide (LiOH) or lithium carbonate (Li2CO3), which achieved a current efficiency of approx. 40 % and a specific energy consumption of 20 kWh/kg. Further optimisation of the cell design is expected to reduce the energy consumption.

Membrane flow cell setup at SINTEF
Additionally, this process also produced a mix of Li2CO3 and LiOH through evaporative crystallisation, with a purity of almost 90 %, but showing sodium (Na) as the main impurity interfering with the process.
The organic-based membrane electrolysis, developed at TEC and tested on three types of solutions – those produced by the liquid-liquid extraction processes from brines and from spodumene leachates, and the off-specification cathode leachates – achieved up to 95 % Li yield, far beyond the levels established at the beginning of the project. Their tested carbonation method yielded a Li2CO3 with a purity exceeding 99% in the case of off-specification cathode material and spodumene concentrate materials. Not only the Li recovery target has been achieved, but also the solvent used in the former leaching process has been recovered and reused keeping the performance as initially, aiming for a more sustainable and circular process.

3-chamber Flow cell setup at TECNALIA
The research group at Fraunhofer Institute for Chemical Technology ICT explored a simple, highly scalable method for lithium purification using a combination of Ion Exchange (IE), Reversed Osmosis (RO) and Electrodialysis with bipolar membranes (EDBM) (see figure below). The goal was to recover high-purity lithium carbonate from Lithium-concentrated solutions provided by partners EnBW and KIT. The IE process effectively removed specific impurities (e.g. divalent cations). The significant level of impurities present in the solutions, provided by EnBW, prevented the electrodialysis with bipolar membranes. The EDBM process, applied uniquely on the samples sent by KIT, yielded a 99.89 % purity. However, the yield of the first precipitation step was determined to be around 35 %, highlighting the need for further optimisation in future precipitation cycles.

Setup to prepare Li2CO3 recovery from Li-concentrated solutions starting with ion exchange, via reversed osmosis and electrodialysis. © FRAUNHOFER
SINTEF researchers investigated the extraction of lithium and other valuable elements, such as Co, Ni, Mn from solid raw materials. They achieved selective chlorination of lithium from calcined spodumene concentrate and off-specification cathode waste in LiCl-KCl and CaCl2-NaCl-KCl melts. Theoretical assessments suggest that lithium can be subsequently electrowon from the LiCl-KCl mixture with a purity of approximately 99 %.

Chlorination apparatus at SINTEF
VITO-ELEC team focused on internally-developed Gas-Diffusion Electrocrystallisation (GDEx) technology, which demonstrated high efficiency – achieving lithium extraction rates more than 95 %. VITO-ELEC team successfully extracted lithium from various sources, including geothermal brines, effluents from sorption processing of hard rock beneficiation and the leachates of off-specification cathode materials.
The team has produced lithium carbonate from the extracted lithium by implementing a downstream synthesis procecure. The process achieved a >1 % lithium concentrate increase from geothermal brines and solid product eluates with over 20 % lithium concentration. Moreover, the energy consumption of the GDEx process was below 10 kWh per kg of Li2CO3, with over 90 % lithium recovery from all tested complex matrices.
Research partners from the LiCORNE consortium are working on developing and optimising various technologies to produce battery-grade materials. SINTEF, for instance, have designed, built and tested their advanced electrodialysis apparatus using purified lithium (Li) solutions derived from the upstream treatment processes of Li-ore. Intermediary results show the process will require further optimisation to obtain 99 % purity LiOH and the targeted energy consumption of less than 15 kWh/kg. The research is still ongoing, focusing now on removing the Al ions prior to the electrodialysis process and on investigating new operating parameters.
In another task, working on the optimisation of the conditions for selective chlorination of spodumene concentrate and cathode waste, SINTEF achieved almost 95 % Li yield using CaCl2 -NaCl -KCl melts. Optimisation is underway to replicate the results to the other valuable materials available in the cathode material.
Researchers at TEC have been optimising the organic-based membrane electrolysis process to recover Li from organic solutions as Li2CO3. Results indicate they managed to achieve over 95 % Li yield from off-specification cathode material, while recovering all the organic solvent used in the previous (leaching) step for its reuse. Good yield rates have also been obtained for the treatment of solutions produced in the liquid/liquid [L/L] extraction of brines and spodumene. However, the selectivity of the membrane is insufficient to overcome the migration of the high concentration of other competing cations such as Na, K, Mg and Ca. Researchers are currently producing and testing new PIMs (Polymer Inclusion Membranes) to try to improve the results.
The research group at VITO have been refining their gas-diffusion electrocrystallisation process for Li recovery from brines, achieving over 95 % removal of Li from most of the samples. By manipulating and adding salts to the brine sample, results show that more than 99 % Li is extracted. The energy efficiency of the GDEx process can be improved with the optimisation of the GDEx reactor.
With all technological processes reporting progress and reaching the targets established at proposal stage, future months will rely on the results of the LCA and LCC analysis, which will establish the most promising processes that will enter the upscaling phase.
© visual:Adobe Stock Photos