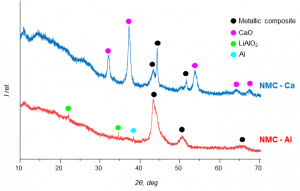
XRD patterns of the MC processed cathode waste materials with Al and Ca as reducing agents
Using alkaline leaching process on spodumene concentrate, the maximum extraction of Li achieved thus far reached 75%. The leachate transformation, even after the filtration step, hinders the sample analysis and further processing. To overcome this challenge, upcoming experiments will explore elevated temperatures, diverse additives, and further investigate the chemical precipitation process.
During the advanced solvometallurgy applied on spodumene concentrate, the research team at TECNALIA reported high Li leaching yields (>95%). Their future work will focus on the further optimisation of the operational conditions, more appropriate for the anticipated scalability phases of the process. On the other hand, solvometallurgical tests carried on waste cathode material achieved high leaching yields for all target elements (Li, Co, Ni, Mn) using mild operational parameters.
After the first experiments engaging reactive milling and aqueous leaching [treated with aluminium- (Al) and calcium (Ca) – salts] on waste cathode material, researchers at KIT reported close to 31% Li recovery rate. Samples supplied by UMICORE were leached under different conditions to extract Li – available in the form of Li carbonate [LiCO3], and further subjected to purifications processes employing various reducing agents. Future efforts for this particular task will focus on adjusting leaching temperatures, establishing an optimal purification process, and evaluating Li recoverability in both Al and Ca systems.
Anticipating future upscaling phases, researchers at VITO, working on the Li-sieve adsorption and desorption from aqueous leachates, shaped the lithium-titanium-oxide (LTO) adsorbents into spheres, which enabled dynamic testing. The team is currently optimising the flow rates for adsorption and desorption to model the optimal conditions for upcoming processes. While initial tests utilised synthetic Li solutions, upcoming research will extend to purification processes for spodumene leachates.
In the same work package, TECNALIA performed experiments using different organic solvents for the liquid/liquid (L/L) extraction from brines showing promising Li yields in the range of 40-60 %.
Within the same work package, EnBW scientific team has been working on designing an eco-friendly Li-desorption process from brines, focusing on the development of novel synthesis for Mn-based adsorbent material. Notably, the successful upscaling of the synthesis process from 2,5g to 200g marks a significant achievement in sustainable material synthesis.
Finally, the last task of WP5 – Electrode-based Li adsorption and desorption from brines, conducted by KIT, presented the conclusions of their research work carried during the last six months, which includes a 4-step process. Their work has been focusing recently on the optimisation of the electrode pre-treatment, the establishment of the current densities and the reduction of the Na contaminations. Despite high Li selectivity rates obtained thus far, their work in the upcoming months will centre around optimising the recovery efficiency and the selectivity. Future experiments will test different thermal operating conditions (40°, 60°, 80°), but will also attempt to scale-up the process.
In the final technical work package, SINTEF scientists are pioneering a two-step process which involves in a primary phase selective chlorination by converting insoluble oxides to soluble chlorides; this is followed by a second step – electrolysis of the soluble chlorides extracting the target elements. After conducting different chlorination experiments, researchers emphasised the importance of time and the processing duration, confirming over 65 % Li recovery rate. With promising results, their focus pivots towards the second step – electrolysis.
Read the next article for a comprehensive overview of the meeting.
Focusing on mechanochemical (MC) processing, the recovery of high-value components from the cathode waste supplied by UMICORE is planned to be performed within Task 4.3.
The ball milling process of waste cathode material was optimised at laboratory scale using different reducing agents such as Al, Ca, and their mixtures. The role of the MC conditions (ball-to-sample ratio (B/S), ball milling time, and nature of the reducing material) was further investigated and analysed. This showed the kinetics of the MC-induced reduction reaction is sensitive to multiple processing parameters.
After the reduction reaction, the powder X-ray diffraction (XRD) analysis revealed the formation of metallic composites and Al/or Ca oxides, as illustrated in the figure below. The upcoming research will be dedicated to investigating and optimising the aqueous leaching conditions of the ball-milled samples at laboratory scale.

XRD patterns of the MC processed cathode waste materials with Al and Ca as reducing agents