- © TU Delft
- © TU Delft
During conventional mineral processing, significant resources are often lost during the beneficiation phase. Lithium-bearing particles trapped in the gangue can proceed to downstream stages, reducing the efficiency of the entire extraction process. To address this, researchers at TU Delft have developed an Opto-Magnetic Sorting System that significantly enhances the separation of lithium ores. This innovative technology combines precision liquid deposition and magnetic separation techniques, offering an advanced alternative to traditional gravity-based separation methods used in beneficiation circuits.
The process starts with lithium-bearing ores being crushed and sieved, isolating particles in the 2–4 mm size range for the next step – optical sorting. A high-resolution line scan camera captures continuous images of particles on a conveyor belt. These images are processed in real-time using a custom algorithm developed at TU Delft, which is trained to identify lithium-rich particles based on subtle colour differences.
Once identified, the target particles are selectively marked using magnetic powder. This enables the marked lithium-rich particles to be separated efficiently by a downstream magnetic conveyor into a dedicated container.
This innovative beneficiation approach has successfully prevented around 45% of the gangue material from entering the downstream process—nearly three times more efficient than the initially targeted improvement of 15%.
According to the State-of-the-Art [SoA], processing spodumene takes place at high-temperatures [1100oC], with direct implications on the economic viability of the entire process. Researchers at TEC have been investigating an alternative to conventional processes. Their investigation features ball milling and calcination at lower temperatures than the conventional process, using additives when needed for the improvement of the next leaching step.
Ball milling is a mechanical process that induces self-sustaining reactions in many sufficiently exothermic powder mixtures. These exothermic reactions, which release a significant amount of heat, can influence both the microscopic and macroscopic properties of the resulting material. On a microscopic level, the heat generated by the reactions can cause changes in the crystal structure and composition of the material. On a macroscopic level, these changes can affect the material’s overall properties, such as its strength, hardness and reactivity. TECNALIA’s findings show that the combination of the ball milling with additives lower calcination temperatures required [200oC below the SoA] in the pre-treatment process of the samples and, also, allow milder conditions in the next processing phases (leaching).
The process, replicated on lithic mica and lithium phosphate materials, were also successful to achieve good results in the next leaching step.

The furnace used in the calcination pre-treatment by TECNALIA
A large group of people arriving on 15 May to Milos island was gathering at Adamantas Conference Centre for the LiCORNE Dissemination Event. A homogenous group of participants, both local authorities and stakeholders from all conrners of Europe, some privileged and attending in person, others online, opened up the floor for discussions around innovative approaches for sustainable extraction of critical raw materials (CRMs) and the role of geothermal fields.
Organised by AdMIRIS on the Milos island, known for its rich geothermal and mineral resources, the event addressed mining sustainability and lithium market dynamics at international, European and national level.
Europe, from its position as an ambassador of the green transition, is expected to see a robust increase in demand for lithium. Although not as well endowed when it comes to lithium as Australia, China and Chile, it is still home to an estimated 5 % of the global reserves. Its insignificant contribution to the global supply highlights the need for strategic reserves and investments in mining and recycling to ensure a stable supply and resilience against market volatility. Presentations at LiCORNE’s Dissemination Event hinted at timely investments in strategic reserves of lithium while prices remain low. This would, ideally, run in parallel with investing in new mines in Europe, incentives for recycling initiatives and continuous development of a performant infrastructure to support the adoption of electric vehicles.
After a brief introduction into the strategic importance of lithium as a critical raw material for green technologies, Dr. Christos Kanellopoulos from the Hellenic Survey of Geology and Mineral Exploration (HSGME), mapped various Li deposits on the map of Greece, along with national exploration projects currently assessing the metal’s presence in various ore deposits – pegmatites, lignite deposits, high salinity closed lakes, geothermal fluids and tertiary basins.
Getting lithium out of European ground is not easy. The metal can be found mostly in hard-rock deposits, which require open pit mines that are usually large, polluting, water-intensive and noisy. Mining projects in Europe are often met with hostile attitudes by the “not-in-my-backyard” and environmental groups. The Greek perception on the mining context in Europe, presented by Mr. Konstantinos Yazitzoglou, Chairman of the Greek Mining Enterprises Association, was both awakening and engaging.
The first take-away set the context, which reminded clearly that all human activities, including mining, create an impact on the environment. Our challenge today is to balance this impact with the benefits it brings. Historically, Western countries have subcontracted mining activities to other parts of the world, often disregarding the environmental and social impacts. Today, this practice is no longer sustainable as those regions are no longer willing to bear the negative consequences.
The BRICS group controls a significant portion of the world’s critical raw materials. With this challenging scenario, Europe has initiated strategic projects and legislative measures to address this issue, but progress has been relatively slow.
The mining industry carries a few ‘negative images’ – including incidents, professional provocateurs and spontaneous reactions from local communities, that should be addressed if Europe aims to resuscitate its mining activities.
To foster a healthy relationship with local communities, finding common grounds on how to disagree and addressing concerns with full transparency remain essential. Emphasising the social dimensions of mining, Mr. Konstantinos Yazitzoglou presented a Greek initiative to create a network of mining township to promote the coexistence of mining and local communities.
During the second part, the LiCORNE Dissemination event spiced up with contradictory presentations. PPC Renewables, operating numerous wind farms, hydroelectric and photovoltaic plants and a hybrid power plant, has recently leased several geothermal concessions in different regions in Greece, including one in Milos-Kimolos-Polyaigos island group. Geophysical surveys and drilling have revealed significant geothermal potential in Milos. Key findings include high conductivity areas in the eastern part of the island, a clear division of the island into two geological sections, and the presence of geothermal fluids in the eastern part of the island.
Despite the richness of the geological formations and the company’s initiatives to engage with the local community, during the event, local authorities made an announcement that no geothermal explorations will take place on the Milos island.
However, the island already has exemplary use cases of mining activities nurtured through social responsibility and engagement with local communities. Imerys is a world leader in mineral-based specialties, providing high-added solutions to various industries, including construction and consumer goods. The company, also a partner in the LiCORNE project, succeeds through performant operations, commercial excellence, market-driven innovation and a strong business model.
In 2018, Imerys launched their SustainAgility programme, structured into three key areas:
Imerys use case reflecting their activities on the Milos island is a sustained effort across several years. Three to four years of corporate involvement in identifying stakeholders, analysing their influence and interests, managing relationships, planning and reporting outcomes through consultation, communication, negotiation, compromise and building relationships that stand the test of time. Imerys presence in Milos, especially during the Covid pandemic, ensured the island’s resilience in times when tourism regular activities were restricted. Imerys long-term operation in Milos relies on balanced development, co-existing with tourism businesses. The company has invested in various initiatives to secure social acceptance and support from the local community.
Various partners in the LiCORNE project presented their innovative research and innovation [R&I] approaches aimed at supporting the sustainable exploration and exploitation of lithium resources. These partners showcased cutting-edge technologies and methodologies designed to minimise environmental impact while maximising resource efficiency, ensuring that lithium extraction aligns with sustainability goals and contributes to the green energy transition.
The LiCORNE project coordinator, Dr. Lourdes Yurramendi opened the technical sessions with an introduction into the scope of work and the objectives, leading after the conversation to the presentations of the specific technologies explored by the LiCORNE partners:
At the LiCORNE EU Project Dissemination event, Nader Akil [Business Operations Manager at PNO] outlined how the EU’s funding is strategically distributed to support R&I initiatives like LiCORNE. The EU’s evolving policy mix, including the Critical Raw Materials Act [CRMA], proposed in March 2023, focuses on ensuring a diverse and secure supply of materials for new industries, setting priorities and benchmarks for 2030. The NetZero Industry Act [NZIA] aims to scale up clean technology manufacturing in the EU to 40 %, with fast-track permitting and strategic projects. The Innovation Fund, closely tied to the NZIA, supports net-zero technologies, including €1 billion for electric vehicle battery cell manufacturing and funding for lithium extraction combined with geothermal installations. The Competitiveness Compass aims to retain Europe’s competitive edge by closing the innovation gap and decarbonising high-impact sectors. With over €22.5 billion in strategic project investments and ambitious 2030 benchmarks, structured innovation ecosystems are essential.
The Upper Rhine Graben (URG) is a promising area for geothermal lithium (Li) extraction, with concentrations in deep geothermal brine exceeding 150 mg/L. They often come accompanied by significant flowrates suitable for geothermal power and heat production. Despite this richness, we still cannot identify with precision the mechanisms leading to the brine enrichment in Li.
ÉS-Géothermie (ÉS-G) researchers characterised the major and trace elements, including Li content, of 34 samples collected from both the Triassic limestone and the sandstone from the Soultz-sous-Forêts wells. They analysed them according to their depth and lithostratigraphy, correlating the Li content with eventual mineralogical changes induced by fluid-rock interactions during diagenesis or hydrothermal circulations. The study suggests that water-rock interactions in both Variscan and Triassic rocks release lithium from phyllosilicates into the brine, which is then trapped in permeable zones, particularly in deep-seated granite.
While lithium concentrations in Muschelkalk and Buntsandstein vary between 1 and 90 ppm, they reach up to 1938 ppm in granite, indicating higher lithium content in Triassic formations. These findings refine the understanding of high lithium concentration in URG brine and highlight specific reservoir zones where lithium enrichment occurs. Future studies will aim to correlate lithium concentrations with fracture characteristics, such as density and aperture, to better understand the relationship between permeability and structural occurrence.
If the subject interests you, ÉS-G researchers will present the findings of their investigations at the European Geothermal Congress, between 6 and 10 October 2025.
On 12 December 2024, the third edition of the annual workshop of the Cluster Hub “Production of Raw Materials for Batteries from European Resources” took place in Brussels, being co-organised by EU-funded projects RHINOCEROS, CRM-geothermal and CICERO. This third edition, along with an increasing number membership, confirm the hub’s role as a dynamic ecosystem that continues to generate innovations in the European battery materials sector.
The hub’s annual workshop, held as a satellite event of the Raw Materials Week 2024, provided once again a platform for presenting the most promising results from participating projects. Two technical sessions covered the entire battery value chain, from raw materials mining to recycling, while the opening conveniently portrayed the policy, the regulatory and strategic frameworks that support and drive the EU R&I initiatives in the battery sector.
Susana Xara, Project adviser on raw materials at European Health and Digital Executive Agency (HaDEA), established the discussions tone, navigating through the insights of the Critical Raw Materials Act [CRMA] and the Net Zero Industry Act [NZIA] and focusing on their contribution to securing a sustainable supply of critical raw materials for the European battery industry.
Wouter IJzermans, BEPA Executive Director, presented the long-term vision and potential revisions of their roadmap, emphasising the importance of policy frameworks and incentives in promoting battery innovation and deployment across Europe.
The presentation of Vasileios Rizos from the Centre for European Policy Studies (CEPS) identified various barriers and challenges emerging from the EU policy framework on batteries, based on inputs from 20 companies across the entire battery value chain, including partners from the BATRAW project, member of the Cluster Hub since 2022. The representative of CEPS concluded with a set of policy messages referring to early dialogue channels established between policy-makers and various stakeholders. Before the legal requirements entry into force, this information exchange on availability of secondary data sets could enable stakeholders to assess the data quality, select suitable sets of information and identify potential data gaps.
Publicly available resources submitted by CEPS:
Orchestrating the launch and on-going work of the Cluster Hub, PNO Innovation Belgium [part of PNO Group – leader in innovation and funding consultancy], represented by Dr. Nader Akil, concluded the first session with an overview of all EU funding programmes supporting research, innovation and investment in raw materials production for batteries. Additional to the upcoming funding opportunities and guidance on selecting the appropriate funding opportunities based on the status of technology, Dr. Nader Akil introduced another initiative launched by PNO Group – DIAMONDS4IF. This project supports the preparation of Innovation Fund applications, enabling the transfer of H2020 research results into successful ventures and securing investment funding.
Download Funding Schemes presentation
The session of technical presentations debuted with RAWMINA project, represented by Carmen Estepa, R&D Manager at AGQ Mining & Bioenergy, providing an overview of its final results on the demonstration activities of an integrated innovative pilot system for CRMs recovery from mine wastes. Up-to-date results indicate encouraging extraction rates of ~90 % Fe, ~95 % Co and ~60 % antimony (Sb) yielded by the bioleaching process. Additionally, the alkaline leaching applied after bioleaching extracted more than 90 % tungsten (W), while the following processing step – Fe precipitation, confirmed that Fe and Sb can be removed almost completely from the solution (>90 %). Finally, the processes engaged in the selective CRM recovery yielded promising recovery rates in the range of 99 % for Co, 65 % for W, 77 % for Sb.
Dr. Albert Genter, Deputy General Manager of ES Géothermie, presented the geochemical characterisation of geothermal reservoir rocks in the Upper Rhine Graben – results of their activities conducted within the LiCORNE EU-funded project. After a short incursion into the geological formation of the Upper Rhine Graben (URG) area, Dr. Albert Genter highlighted the feasibility of lithium (Li) extraction from the geothermal brines. The high Li concentrations in the geothermal brine at Soultz-sous-Forêts and Rittershoffen [in the range of 150-200 mg/L], combined with significant water flows exploited by the geothermal power plants, indicate a great potential for Li production in the URG. After establishing the fluid circulation within the fractures of the geological formation, the research team at ES-G will continue investigating the chemical composition of sedimentary rocks, which are also part of the reservoir Soultz-sous-Forêts, and conducting Li and strontium (Sr) isotope analyses to provide more detailed information about the origin of lithium in the brine.
Dr. Nivea Magalhães [Univ. of Exeter, UK] presented the conclusions of the forensic geometallurgy protocol established within the ENICON project. Often, information not directly related to processing leads to limited insights into ore processing behaviour. ENICON investigates the impact of mineral textures and grain size on liberation, sometimes interfering with automated mineralogy results. Additionally, the project presented the findings of the ore characterisation of the Kevitsa mine, containing nickel (Ni)- and cobalt (Co)-bearing minerals.
The CRM-geothermal presentation, delivered by Saskia Bindschedler, Professor at Univ. de Neuchatel, Faculty of science, Institute of biology, Laboratory of microbiology, focused on the use of microbial activity for Li recovery from geothermal brines. Geothermal brines are characterised by high temperatures, increased pressure and salinity, conditions favourable for bioextraction processes using microbes. Key findings confirmed the feasibility of microbial-driven processes for Li recovery, enabling effective filtering of elements using oxalate compounds, followed by precipitation via oxalothropic bacteria, such as Pandoraea sp.. While the researchers will continue working on oxalotrophy and initial pH optimisation, focusing on improving the scalability, they will additionally investigate Li concentration in fluid samples.
Download CRM-geothermal presentation
An insight into the results of the METALLICO project, with focus on their COOL+ technology, was delivered by Sandra Pavon from Fraunhofer IKTS. COOL+ is one of the five technologies investigated within the framework of METALLICO, that involves a leaching step using supercritical CO2, that enables the extraction of Li in a more efficient and environmentally friendly manner. After explaining the five phases of the process and comparing the results at the main conclusions reported high selectivity and efficiency in Li recovery, achieving Li2CO3 which meets battery-grade specifications with a purity of 99.7 %. The solid silicate residue that remains after the CO2 leaching step is not wasted. Instead, it is repurposed to produce geopolymers which are further used in the construction sector, aligning with the principles of circular economy and zero-waste.
Download METALLICO presentation
Partners working on various extraction processes of lithium (Li) from a variety of feedstocks – concentrates, waste cathode material, ore and tailings, are reaching target recovery rates.
NTUA researchers have developed an alkaline leaching process to extract Li from spodumene concentrates, which yielded extraction rates of over 92 % and low impurities. The same process, this time applied on lithic mica, resulted in Li extraction rates of nearly 100 % at a longer leaching duration. Moreover, the optimal settings showed the capacity to maintain the level of impurities low. Leaching experiments on mica samples will continue, but results are already encouraging. This new leaching process requires temperatures considerably lower than the conventional extraction process, currently at 1100°C.
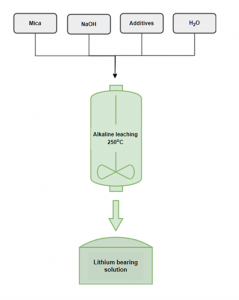
Alkaline leaching scheme to extract lithium NTUA
On their side, researchers at TEC have been optimising the solvometallurgical process to extract valuable elements from four type of materials: spodumene concentrates, lithic mica, lithium phosphate and off specification cathode material. After achieving their target of more than 95 % Li extraction from spodumene, the optimisation phase tested (taking advantage of the result from the novel pre-treatment established and previously described) milder leaching conditions, obtaining similar good results. For lithic mica and lithium phosphates, best operations routes investigated have shown that pre-treatment increases considerably the leaching yield at values higher than the target. For the off-specification cathode material, researchers have concluded that mechanical activation of the cake obtained after leaching improves Li extraction, achieving up to 99 % Li recovery and very high selectivity at room temperature processing. Ni, Co and Mn can be separated as a valuable mixture in the same process.
Finally, the research team at KIT, in charge of the reactive milling and aqueous leaching of waste cathode material [NMC], optimised the purification processes using various reducing agents. The intermediary results yielded Li recovery rates ranging between 68,8 % and 91 %, depending on the reducing agent utilised during the purification process. Next steps for KIT research group expand to calculating the lithium carbonate [Li2CO3] purity, determining the recovery rate of Ni, Mn and Co and upscaling the ball-milling.
VITO researchers, working on the Li-sieve adsorption and desorption from aqueous leachates, shaped the lithium-titanium-oxide (LTO) adsorbents into spheres, which enabled dynamic testing. The optimised flow rates and settings initially modelled on synthetic Li solutions have been recently tested on real samples, yielding approx. 85 % Li recovery from aqueous alkaline spodumene leachates. The team at VITO has recently filed a patent application with the desorption stability results.
Expected results have already been shaping up in Spain, where TEC is working on the Li extraction from both continental and geothermal brines. After running tests using the most suitable extractants for their liquid-liquid extraction process [L-L] coupled with stripping operations, researchers have managed to obtain a global Li extraction of 92 % from continental brine, far beyond the initial target of 85 %, while diminishing the content of the accompanying cations (Na, K, Ca and Mg). On the other hand, the same technological process applied to spodumene yields a global recovery rate around >90 % after optimisation of the scenarios based on McCabe-Thiele diagrams.
In another European region, famous for its geothermal resources, EnBW researchers have been investigating Li-extraction from brines. They developed a novel synthesis route for Lithium Manganese Oxide [LMO] adsorbent, for which a patent has been recently filed. The LMO adsorbents have been demonstrating high absorption capacity and selectivity for Li extraction from brines with high salt contents. Offering improved chemical stability and potential for large-scale production of the material, this solution looks very promising for future implementation at industrial level for Li recovery.
Another extraction process, the electrode-based Li adsorption and desorption from brines, has been optimised by KIT. Following the principles of a salt water battery, the electrochemical extractions with Li-selective electrodes yielded encouraging results for the Li-extraction from geothermal brines. The Li selectivity in the recovery solution were in the range of 77 % to 82 %, displaying a good separation from the main contaminants.
© visual: TECNALIA
During the M24 consortium meeting held in Karlsruhe (GER), the project team presented the latest progress achieved in the work package dedicated to the supply and characterisation of the feedstock, with a primary focus on the geochemical analysis of geothermal brines and rocks.
Between M18 and M24, researchers collected and sent for analyses geothermal brine from the reservoir at Soultz-sous-Forêts in France. This latest analysis not only revealed a Li concentration above 170 mg/L, which confirms the stability and the quality of this resource for a potential future lithium extraction in the Upper Rhine Graben geothermal brine.
In addition to brine analysis, the researchers conducted thorough geochemical analyses on core samples from three deep wells in Soultz-sous-Forêts. These wells intersect the Muschelkalk limestone, Buntsandstein sandstone and Visean granite formations. A total of 57 core samples, sourced from depths ranging between 841 to 5060 m were selected for analysis. The focus was on 36 granite samples, where the lithium concentrations varied significantly. According to the analysis of the research team at ES-G, Li concentrations tend to be highly impacted by hydrothermal alteration. They found that Li concentration can vary by two orders of magnitude when compared to the fresh granite mainly due to secondary minerals precipitation. However, solubilisation of Li is identified in most of the case where hydrothermal alteration is important.
Stakeholders interested in the characterisation performed by ES-G have the chance to find more detailed information at the upcoming Stanford Geothermal Workshop, taking place between 10 and 12 February 2025.
Further isotopic analysis of Li and Sr in rock samples will allow researchers to further understand the sources and mobilisation of Li in geothermal brines. These analyses will provide more accurate insights into the geochemical processes involved and support the development of more efficient and sustainable lithium extraction methods.
© visual:Adobe Stock Photos
On 16 October 2024, the Karlsruhe Institute of Technology (KIT) was hosting not only the LiCORNE project’s M24 consortium meeting, but also its first exploitation workshop. The event brought together a diverse group of stakeholders, with nearly 15 industry guests and members of the External Advisory Board (EAB), to discuss the latest advancements in lithium (Li) extraction technologies.
The workshop began with a welcome address by Dr. Lourdes Yurramendi [the coordinator of the LiCORNE initiative and Project Director at TECNALIA Waste Valorisation, Energy, Climate and Urban Transition], followed by Nader Akil, Operations Manager at PNO Innovation Belgium, who outlines the objectives of the exploitation workshop and provided an overview of the LiCORNE project. Funded by the European Commission, the project aims to develop competitive technologies for Li extraction and recovery from various feedstocks, including ores, geothermal brines and cathode waste materials. Following this introduction, various partners delivered technical presentations, showcasing their innovative approaches and key exploitable results after 24 months from the start of the project.
Regardless the feedstock considered, all these novel technologies share one theme: sustainability. This focus on sustainability translates into exploring research routes that go beyond the current state-of-the-art (SoA), reducing energy and water consumption and the generation of chemical waste:
Beyond technological presentations, the workshop also facilitated discussions with external participants, including members of the EAB and industry representatives. These exchanges provided valuable insights into the industry’s needs and opened up new routes for collaboration. To facilitate future collaborations, PNO presented several funding opportunities that can be used to bring the most promising technologies and the LiCORNE selected flowsheet to a pilot level.
As the project progresses, the focus will shift now towards the benchmarking and selection of the most promising LiCORNE technologies for upscaling to produce ~1 kg of battery-grade Li by the end of the project. This phase aims to shape a path towards larger piloting and future commercialisation.
Author: ÉS-GÉOTHERMIE [ÉS-G]
Among European geothermal sites, the Upper Rhine Graben (URG) has a great potential for a lithium (Li) production from geothermal brines due to its high concentration and the significant water flows exploited by the geothermal power plants in this area.
Despite its great potential, certain gaps in the basic knowledge of the geochemistry of the URG rocks are persisting, as there is scarce conclusive investigation carried out in the past to estimate the Li content as well as the mechanisms of Li recharge in brine. Identifying Li-rich geological units are essential to target areas with higher Li concentrations for exploration and to ensure the sustainability of this resource.
In geothermal systems, hydrothermal fluids circulate through the fractured and porous rock formations, undergoing complex interactions with the surrounding lithology. Various processes, such as leaching, dissolution, and precipitation, can occur and they can significantly influence the concentration of Li in the circulating fluids. Knowing the chemistry of the reservoir rocks could help us understand chemical reactions occurring between the hydrothermal fluids and the rocks and therefore how Li is mobilised and transported into the geothermal brine.
In the LiCORNE project, ESG is conducting detailed geochemical analysis of several core drills including granite, sandstone, and limestone from geothermal wells drilled in Northern Alsace. Researchers finalised the rock sampling task at the beginning of 2024, while the chemical measurements are expected at the end of June, current year.

Sampling of granite rocks in the core shelter. © ES-Géothermie (ESG)
In total, 57 samples were collected and closely studied, which facilitates understanding of the chemical elements behaviour in the rock before and after the hydrothermal circulation/alteration. Comparing the results of this on-going investigation with the few data available in literature and referring to the Li concentration in URG rocks could reveal an unexpected behaviour of Li in the geothermal reservoir rocks.
After careful analysis of the chemical composition, isotopic analysis of the same rock will follow which will show more accurately potential sources of Li in the geothermal brine.
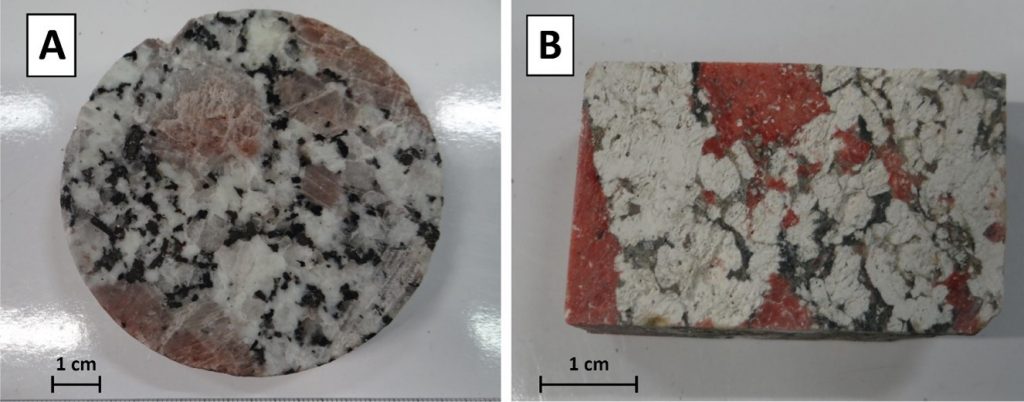
A. Fresh monzogranite sampled at 1774.5 m depth); B. Hydrothermally altered granite showing argillic alteration sampled at 2159.30 m depth. © ES-Géothermie (ESG)
The most recent political events and regulatory context have highlighted on several occasions a growing demand for lithium (Li), primarily fuelled by the increasing use of lithium-ion batteries (LIBs). The concentration of the largest mining sites for Li outside of Europe leads to a strong dependence on third countries, which can pose economic and strategic challenges for the EU. To mitigate the resources scarcity, the EU has implemented regulations promoting sustainable battery practices, and is actively exploring diversified domestic resources of Li, such as the geothermal brines located in the Rhine Graben region.
Researchers at Fraunhofer ICT recently attended the German Geothermal Conference in Essen (Germany), where they submitted a scientific poster that portrays the characteristics of the Li resources identified in the geothermal brines in the Rhine Graben region and the challenges raised by the Li extraction. Due to the high concentration of salt load, the selective separation of Li and sodium (Na) remains the primary challenge to solve. Within the LiCORNE project, researchers are testing a manganese-based (Mn-based) adsorption setup to provide Li enriched solutions from geothermal brines. Despite recent developments, impurities persist, and additional separation steps are required.
During the German Geothermal Conference, the research team at Fraunhofer ICT presented the free-flow electrophoresis (FFE) used for the selective separation of Li and Na ions. This separation relies on the ions’ migration velocity in an electrified field, being significantly influenced by their charge, size and hydrate shell.
The main advantage of the FFE lies in its capacity to prevent mixing of individual streams with the background eluent, allowing separate collection of the individual streams at the end of the chamber. Additional benefits of the FFE:
Testing the method with different parameters, various concentrations of the sample solution and altering the eluent solution, researchers reported a complete separation of Li and Na ions by FFE, with over 80% separation efficiency. Future efforts will focus on testing the actual desorption solutions, optimizing throughput, scaling up, and reducing costs.
Download the original poster, available in German, here