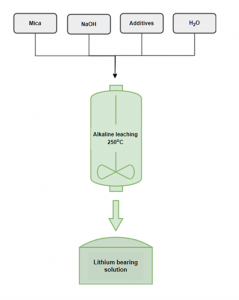
Alkaline leaching scheme to extract lithium NTUA
On 12 December 2024, the third edition of the annual workshop of the Cluster Hub “Production of Raw Materials for Batteries from European Resources” took place in Brussels, being co-organised by EU-funded projects RHINOCEROS, CRM-geothermal and CICERO. This third edition, along with an increasing number membership, confirm the hub’s role as a dynamic ecosystem that continues to generate innovations in the European battery materials sector.
The hub’s annual workshop, held as a satellite event of the Raw Materials Week 2024, provided once again a platform for presenting the most promising results from participating projects. Two technical sessions covered the entire battery value chain, from raw materials mining to recycling, while the opening conveniently portrayed the policy, the regulatory and strategic frameworks that support and drive the EU R&I initiatives in the battery sector.
Susana Xara, Project adviser on raw materials at European Health and Digital Executive Agency (HaDEA), established the discussions tone, navigating through the insights of the Critical Raw Materials Act [CRMA] and the Net Zero Industry Act [NZIA] and focusing on their contribution to securing a sustainable supply of critical raw materials for the European battery industry.
Wouter IJzermans, BEPA Executive Director, presented the long-term vision and potential revisions of their roadmap, emphasising the importance of policy frameworks and incentives in promoting battery innovation and deployment across Europe.
The presentation of Vasileios Rizos from the Centre for European Policy Studies (CEPS) identified various barriers and challenges emerging from the EU policy framework on batteries, based on inputs from 20 companies across the entire battery value chain, including partners from the BATRAW project, member of the Cluster Hub since 2022. The representative of CEPS concluded with a set of policy messages referring to early dialogue channels established between policy-makers and various stakeholders. Before the legal requirements entry into force, this information exchange on availability of secondary data sets could enable stakeholders to assess the data quality, select suitable sets of information and identify potential data gaps.
Publicly available resources submitted by CEPS:
Orchestrating the launch and on-going work of the Cluster Hub, PNO Innovation Belgium [part of PNO Group – leader in innovation and funding consultancy], represented by Dr. Nader Akil, concluded the first session with an overview of all EU funding programmes supporting research, innovation and investment in raw materials production for batteries. Additional to the upcoming funding opportunities and guidance on selecting the appropriate funding opportunities based on the status of technology, Dr. Nader Akil introduced another initiative launched by PNO Group – DIAMONDS4IF. This project supports the preparation of Innovation Fund applications, enabling the transfer of H2020 research results into successful ventures and securing investment funding.
Download Funding Schemes presentation
The session of technical presentations debuted with RAWMINA project, represented by Carmen Estepa, R&D Manager at AGQ Mining & Bioenergy, providing an overview of its final results on the demonstration activities of an integrated innovative pilot system for CRMs recovery from mine wastes. Up-to-date results indicate encouraging extraction rates of ~90 % Fe, ~95 % Co and ~60 % antimony (Sb) yielded by the bioleaching process. Additionally, the alkaline leaching applied after bioleaching extracted more than 90 % tungsten (W), while the following processing step – Fe precipitation, confirmed that Fe and Sb can be removed almost completely from the solution (>90 %). Finally, the processes engaged in the selective CRM recovery yielded promising recovery rates in the range of 99 % for Co, 65 % for W, 77 % for Sb.
Dr. Albert Genter, Deputy General Manager of ES Géothermie, presented the geochemical characterisation of geothermal reservoir rocks in the Upper Rhine Graben – results of their activities conducted within the LiCORNE EU-funded project. After a short incursion into the geological formation of the Upper Rhine Graben (URG) area, Dr. Albert Genter highlighted the feasibility of lithium (Li) extraction from the geothermal brines. The high Li concentrations in the geothermal brine at Soultz-sous-Forêts and Rittershoffen [in the range of 150-200 mg/L], combined with significant water flows exploited by the geothermal power plants, indicate a great potential for Li production in the URG. After establishing the fluid circulation within the fractures of the geological formation, the research team at ES-G will continue investigating the chemical composition of sedimentary rocks, which are also part of the reservoir Soultz-sous-Forêts, and conducting Li and strontium (Sr) isotope analyses to provide more detailed information about the origin of lithium in the brine.
Dr. Nivea Magalhães [Univ. of Exeter, UK] presented the conclusions of the forensic geometallurgy protocol established within the ENICON project. Often, information not directly related to processing leads to limited insights into ore processing behaviour. ENICON investigates the impact of mineral textures and grain size on liberation, sometimes interfering with automated mineralogy results. Additionally, the project presented the findings of the ore characterisation of the Kevitsa mine, containing nickel (Ni)- and cobalt (Co)-bearing minerals.
The CRM-geothermal presentation, delivered by Saskia Bindschedler, Professor at Univ. de Neuchatel, Faculty of science, Institute of biology, Laboratory of microbiology, focused on the use of microbial activity for Li recovery from geothermal brines. Geothermal brines are characterised by high temperatures, increased pressure and salinity, conditions favourable for bioextraction processes using microbes. Key findings confirmed the feasibility of microbial-driven processes for Li recovery, enabling effective filtering of elements using oxalate compounds, followed by precipitation via oxalothropic bacteria, such as Pandoraea sp.. While the researchers will continue working on oxalotrophy and initial pH optimisation, focusing on improving the scalability, they will additionally investigate Li concentration in fluid samples.
Download CRM-geothermal presentation
An insight into the results of the METALLICO project, with focus on their COOL+ technology, was delivered by Sandra Pavon from Fraunhofer IKTS. COOL+ is one of the five technologies investigated within the framework of METALLICO, that involves a leaching step using supercritical CO2, that enables the extraction of Li in a more efficient and environmentally friendly manner. After explaining the five phases of the process and comparing the results at the main conclusions reported high selectivity and efficiency in Li recovery, achieving Li2CO3 which meets battery-grade specifications with a purity of 99.7 %. The solid silicate residue that remains after the CO2 leaching step is not wasted. Instead, it is repurposed to produce geopolymers which are further used in the construction sector, aligning with the principles of circular economy and zero-waste.
Download METALLICO presentation
Research partners from the LiCORNE consortium are working on developing and optimising various technologies to produce battery-grade materials. SINTEF, for instance, have designed, built and tested their advanced electrodialysis apparatus using purified lithium (Li) solutions derived from the upstream treatment processes of Li-ore. Intermediary results show the process will require further optimisation to obtain 99 % purity LiOH and the targeted energy consumption of less than 15 kWh/kg. The research is still ongoing, focusing now on removing the Al ions prior to the electrodialysis process and on investigating new operating parameters.
In another task, working on the optimisation of the conditions for selective chlorination of spodumene concentrate and cathode waste, SINTEF achieved almost 95 % Li yield using CaCl2 -NaCl -KCl melts. Optimisation is underway to replicate the results to the other valuable materials available in the cathode material.
Researchers at TEC have been optimising the organic-based membrane electrolysis process to recover Li from organic solutions as Li2CO3. Results indicate they managed to achieve over 95 % Li yield from off-specification cathode material, while recovering all the organic solvent used in the previous (leaching) step for its reuse. Good yield rates have also been obtained for the treatment of solutions produced in the liquid/liquid [L/L] extraction of brines and spodumene. However, the selectivity of the membrane is insufficient to overcome the migration of the high concentration of other competing cations such as Na, K, Mg and Ca. Researchers are currently producing and testing new PIMs (Polymer Inclusion Membranes) to try to improve the results.
The research group at VITO have been refining their gas-diffusion electrocrystallisation process for Li recovery from brines, achieving over 95 % removal of Li from most of the samples. By manipulating and adding salts to the brine sample, results show that more than 99 % Li is extracted. The energy efficiency of the GDEx process can be improved with the optimisation of the GDEx reactor.
With all technological processes reporting progress and reaching the targets established at proposal stage, future months will rely on the results of the LCA and LCC analysis, which will establish the most promising processes that will enter the upscaling phase.
© visual:Adobe Stock Photos
Partners working on various extraction processes of lithium (Li) from a variety of feedstocks – concentrates, waste cathode material, ore and tailings, are reaching target recovery rates.
NTUA researchers have developed an alkaline leaching process to extract Li from spodumene concentrates, which yielded extraction rates of over 92 % and low impurities. The same process, this time applied on lithic mica, resulted in Li extraction rates of nearly 100 % at a longer leaching duration. Moreover, the optimal settings showed the capacity to maintain the level of impurities low. Leaching experiments on mica samples will continue, but results are already encouraging. This new leaching process requires temperatures considerably lower than the conventional extraction process, currently at 1100°C.

Alkaline leaching scheme to extract lithium NTUA
On their side, researchers at TEC have been optimising the solvometallurgical process to extract valuable elements from four type of materials: spodumene concentrates, lithic mica, lithium phosphate and off specification cathode material. After achieving their target of more than 95 % Li extraction from spodumene, the optimisation phase tested (taking advantage of the result from the novel pre-treatment established and previously described) milder leaching conditions, obtaining similar good results. For lithic mica and lithium phosphates, best operations routes investigated have shown that pre-treatment increases considerably the leaching yield at values higher than the target. For the off-specification cathode material, researchers have concluded that mechanical activation of the cake obtained after leaching improves Li extraction, achieving up to 99 % Li recovery and very high selectivity at room temperature processing. Ni, Co and Mn can be separated as a valuable mixture in the same process.
Finally, the research team at KIT, in charge of the reactive milling and aqueous leaching of waste cathode material [NMC], optimised the purification processes using various reducing agents. The intermediary results yielded Li recovery rates ranging between 68,8 % and 91 %, depending on the reducing agent utilised during the purification process. Next steps for KIT research group expand to calculating the lithium carbonate [Li2CO3] purity, determining the recovery rate of Ni, Mn and Co and upscaling the ball-milling.
VITO researchers, working on the Li-sieve adsorption and desorption from aqueous leachates, shaped the lithium-titanium-oxide (LTO) adsorbents into spheres, which enabled dynamic testing. The optimised flow rates and settings initially modelled on synthetic Li solutions have been recently tested on real samples, yielding approx. 85 % Li recovery from aqueous alkaline spodumene leachates. The team at VITO has recently filed a patent application with the desorption stability results.
Expected results have already been shaping up in Spain, where TEC is working on the Li extraction from both continental and geothermal brines. After running tests using the most suitable extractants for their liquid-liquid extraction process [L-L] coupled with stripping operations, researchers have managed to obtain a global Li extraction of 92 % from continental brine, far beyond the initial target of 85 %, while diminishing the content of the accompanying cations (Na, K, Ca and Mg). On the other hand, the same technological process applied to spodumene yields a global recovery rate around >90 % after optimisation of the scenarios based on McCabe-Thiele diagrams.
In another European region, famous for its geothermal resources, EnBW researchers have been investigating Li-extraction from brines. They developed a novel synthesis route for Lithium Manganese Oxide [LMO] adsorbent, for which a patent has been recently filed. The LMO adsorbents have been demonstrating high absorption capacity and selectivity for Li extraction from brines with high salt contents. Offering improved chemical stability and potential for large-scale production of the material, this solution looks very promising for future implementation at industrial level for Li recovery.
Another extraction process, the electrode-based Li adsorption and desorption from brines, has been optimised by KIT. Following the principles of a salt water battery, the electrochemical extractions with Li-selective electrodes yielded encouraging results for the Li-extraction from geothermal brines. The Li selectivity in the recovery solution were in the range of 77 % to 82 %, displaying a good separation from the main contaminants.
© visual: TECNALIA
Within the beneficiation process, the research group at TUD developed an opto-magnetically induced sorting technology. Within the next months, their work will continue developing their code to optimise the colour identification of target metals, and simultaneously on various set-ups to improve the magnetic attraction and and to ensure the seamless integration of all components of their opto-magnetically induced sorter.
Within the same work package, researchers at NTUA have developed a new calcination technology with additives, tested on spodumene concentrates. Using different settings and parameters, such as the processing temperature, reaction time, pressure, the extraction yields for Li ranged between 71 % and 96 %. Depending on the additive type, adjusting the calcination parameters accordingly can significantly reduce impurities, such as aluminum (Al), present in spodumene concentrate. Simultaneously, NTUA partners have been optimising a new technology for Li extraction with calcination from lithic mica and the results will be available in the upcoming communications.
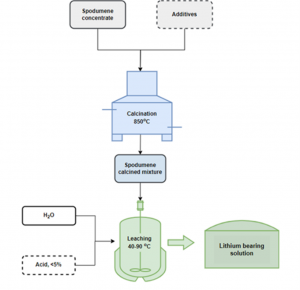
Calcination scheme NTUA
Working on spodumene concentrates, the research group at TEC has established a novel pre-treatment process that allows a relevant improvement in the next leaching process of lithium for its valorisation. As a result of this method, which includes ball milling combined with additive, the transformation of the mineralogical structure of the spodumene takes place at a significantly reduced temperature, ranging from 1100ºC to 900ºC. Based on these findings, TEC has applied a similar approach for the lithium phosphate and the lithic mica materials, reporting good results.
© visual:Adobe Stock Photos
With no surprise, after Europe’s quest to replace fossil fuels and turn towards clean energy, lithium (Li) has been classified as a key component, making it to the short list of EU’s highly significant critical raw materials. With the transition to zero-emission vehicles, carmakers, as the most consuming industrial sector, will need ever more Li for batteries.
Renowned for its policy background, the EU decisional institutions adopted the Critical Raw Materials Act (CRMA) in record time. This accelerated adoption procedure shows nothing but the need for action, which reflects Europe’s urge to secure a sustainable supply of critical raw materials (CRMs). The CRMA sets specific targets to strengthen the EU’s capacities along the different stages of the value chain, ensuring that by 2030:
Both EnBW and LevertonHELM are key partners in the LiCORNE project. EnBW, as one of the largest energy supply companies in Germany and Europe, has the following tasks in the LiCORNE project: 1) to supply geothermal brine feedstock, respectively to conduct develop Li+ desorption technology aiming at min. 90% yield from geothermal and continental brines. LevertonHELM, on the other side, is a Lithium chemicals producer based in the UK, focusing on the manufacturing of a wide range of inorganic Li chemicals. In the framework of LiCORNE, the British company will benchmark and qualify the Li produced by the processes developed in the project, as battery-grade material.
German, respectively British companies have expanded their collaboration beyond the project’s framework, with a joint objective to advance the sustainable production of battery-grade Li carbonate and Li hydroxide – essential materials for electric mobility and energy storage solutions.
In previous articles, EnBW reported high Li concentrations for the geothermal brines in the Upper Rhine Valley (Bruchsal reservoir), ranging from 163 to 190 mg/L (Sanjuan et al., 2016). However, due to the characteristics of the reservoir, featuring highly mineralised brines, the extraction process was hampered by an elevated additional concentration of foreign ions (TDS 130 g/l). According to Laura Herrmann, Project Manager Research and Development at EnBW, the process requires increased selective adsorption technology in line with the exigences of the battery materials producers.
This industrial collaboration has resulted in a remarkable purity of 99.5% for lithium carbonate, demonstrating great potential for further scale-up to meet the EU’s demand for lithium.
Using direct Li extraction by adsorption (A-DLE), the process coordinated by the industrial partnership led to a remarkable purity of 99.5 % for Li carbonate. This successful initial trial holds promise for future upscale, potentially meeting the EU’s demand for Li.
Register to the Symposium on Direct Lithium Extraction
© visual:EnBW
Author: ÉS-GÉOTHERMIE [ÉS-G]
Among European geothermal sites, the Upper Rhine Graben (URG) has a great potential for a lithium (Li) production from geothermal brines due to its high concentration and the significant water flows exploited by the geothermal power plants in this area.
Despite its great potential, certain gaps in the basic knowledge of the geochemistry of the URG rocks are persisting, as there is scarce conclusive investigation carried out in the past to estimate the Li content as well as the mechanisms of Li recharge in brine. Identifying Li-rich geological units are essential to target areas with higher Li concentrations for exploration and to ensure the sustainability of this resource.
In geothermal systems, hydrothermal fluids circulate through the fractured and porous rock formations, undergoing complex interactions with the surrounding lithology. Various processes, such as leaching, dissolution, and precipitation, can occur and they can significantly influence the concentration of Li in the circulating fluids. Knowing the chemistry of the reservoir rocks could help us understand chemical reactions occurring between the hydrothermal fluids and the rocks and therefore how Li is mobilised and transported into the geothermal brine.
In the LiCORNE project, ESG is conducting detailed geochemical analysis of several core drills including granite, sandstone, and limestone from geothermal wells drilled in Northern Alsace. Researchers finalised the rock sampling task at the beginning of 2024, while the chemical measurements are expected at the end of June, current year.

Sampling of granite rocks in the core shelter. © ES-Géothermie (ESG)
In total, 57 samples were collected and closely studied, which facilitates understanding of the chemical elements behaviour in the rock before and after the hydrothermal circulation/alteration. Comparing the results of this on-going investigation with the few data available in literature and referring to the Li concentration in URG rocks could reveal an unexpected behaviour of Li in the geothermal reservoir rocks.
After careful analysis of the chemical composition, isotopic analysis of the same rock will follow which will show more accurately potential sources of Li in the geothermal brine.
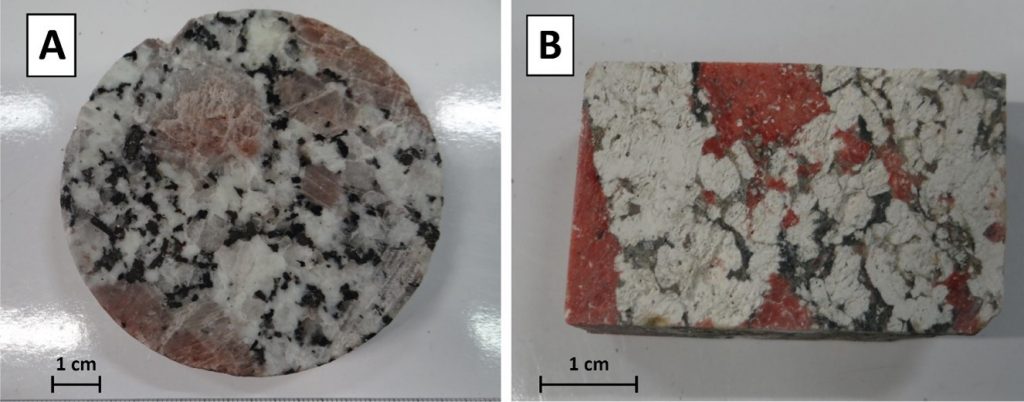
A. Fresh monzogranite sampled at 1774.5 m depth); B. Hydrothermally altered granite showing argillic alteration sampled at 2159.30 m depth. © ES-Géothermie (ESG)
Using alkaline leaching process on spodumene concentrate, the maximum extraction of Li achieved thus far reached 75%. The leachate transformation, even after the filtration step, hinders the sample analysis and further processing. To overcome this challenge, upcoming experiments will explore elevated temperatures, diverse additives, and further investigate the chemical precipitation process.
During the advanced solvometallurgy applied on spodumene concentrate, the research team at TECNALIA reported high Li leaching yields (>95%). Their future work will focus on the further optimisation of the operational conditions, more appropriate for the anticipated scalability phases of the process. On the other hand, solvometallurgical tests carried on waste cathode material achieved high leaching yields for all target elements (Li, Co, Ni, Mn) using mild operational parameters.
After the first experiments engaging reactive milling and aqueous leaching [treated with aluminium- (Al) and calcium (Ca) – salts] on waste cathode material, researchers at KIT reported close to 31% Li recovery rate. Samples supplied by UMICORE were leached under different conditions to extract Li – available in the form of Li carbonate [LiCO3], and further subjected to purifications processes employing various reducing agents. Future efforts for this particular task will focus on adjusting leaching temperatures, establishing an optimal purification process, and evaluating Li recoverability in both Al and Ca systems.
Anticipating future upscaling phases, researchers at VITO, working on the Li-sieve adsorption and desorption from aqueous leachates, shaped the lithium-titanium-oxide (LTO) adsorbents into spheres, which enabled dynamic testing. The team is currently optimising the flow rates for adsorption and desorption to model the optimal conditions for upcoming processes. While initial tests utilised synthetic Li solutions, upcoming research will extend to purification processes for spodumene leachates.
In the same work package, TECNALIA performed experiments using different organic solvents for the liquid/liquid (L/L) extraction from brines showing promising Li yields in the range of 40-60 %.
Within the same work package, EnBW scientific team has been working on designing an eco-friendly Li-desorption process from brines, focusing on the development of novel synthesis for Mn-based adsorbent material. Notably, the successful upscaling of the synthesis process from 2,5g to 200g marks a significant achievement in sustainable material synthesis.
Finally, the last task of WP5 – Electrode-based Li adsorption and desorption from brines, conducted by KIT, presented the conclusions of their research work carried during the last six months, which includes a 4-step process. Their work has been focusing recently on the optimisation of the electrode pre-treatment, the establishment of the current densities and the reduction of the Na contaminations. Despite high Li selectivity rates obtained thus far, their work in the upcoming months will centre around optimising the recovery efficiency and the selectivity. Future experiments will test different thermal operating conditions (40°, 60°, 80°), but will also attempt to scale-up the process.
In the final technical work package, SINTEF scientists are pioneering a two-step process which involves in a primary phase selective chlorination by converting insoluble oxides to soluble chlorides; this is followed by a second step – electrolysis of the soluble chlorides extracting the target elements. After conducting different chlorination experiments, researchers emphasised the importance of time and the processing duration, confirming over 65 % Li recovery rate. With promising results, their focus pivots towards the second step – electrolysis.
Read the next article for a comprehensive overview of the meeting.
Lithium (Li), a highly versatile element, finds extensive applications in diverse industries including ceramics, glass, fuel cells, metallurgy, pharmaceuticals, aerospace, and lithium-ion batteries (LIBs). With the demand of LIBs increasing, particularly fueled by portable electronics and electric vehicles, the global lithium industry is undergoing rapid expansion. Due to its lightweight and reactive properties, Li is considered the essential component in high-energy-density batteries, playing a crucial role in the future of sustainable energy. But the extraction of Li resources has become a critical concern.
VITO employs an innovative process known as Gas-Diffusion Electrocrystallisation (GDEx) technology to achieve the direct extraction of Li, that leverages gas-diffusion electrodes to orchestrate a meticulously controlled chemical transformation. By precisely manipulating its parameters, GDEx enables synthesis with minimal chemical additives, marking a significant milestone in this field. The unique design of the reactor maintains a consistent set of conditions, and by simply altering the inlet solution, it can produce the desired target structure. This approach is highly scalable and promising for the future of Li extraction and synthesis.
Following comprehensive investigations performed on synthetic solutions, VITO fine-tuned the operational parameters to be applicable to natural brine solutions and leachates. The experiments carried out on diverse brine solutions yielded remarkable results, with a Li removal efficiency exceeding 95% from these solutions. The planned process involves producing layered double hydroxide structures which can further be downstreamed to battery cathode material.
Learn more about the GDEx process in the previous article: Recovery as battery-grade chemicals
©Adobe Stock Photos, Salinas Grandes, a huge salt flat in Jujuy and Salta, Argentina.
In July 2023, International Energy Agency (IEA) released its inaugural “Critical Minerals Market Review”, along with their new online data explorer. Between 2017 and 2022, the demand of lithium (Li) tripled, primarily due to the energy sector’s reliance on it. According to the report, the market for energy transition minerals is poised for continued rapid growth, placing increasing pressure on the global mining industry.
Looking in particular at the Li price fluctuations, the study reports increases in 2021 and early 2022, accompanied by strong volatility. However, the latter half of 2022 and the beginning of 2023 saw more stable trends, albeit still remaining above historical averages.
Not unexpected, investment in the development of critical minerals, particularly Li, recorded a significant surge of 30% in 2022, building upon a previous increase of 20% in 2021. The IEA analysis examined the investment patterns of 20 major mining companies actively involved in the production of minerals essential for the energy transition. It revealed a substantial rise in capital expenditure specifically allocated to critical minerals. This upward trend can be attributed to the favourable momentum propelling the adoption of clean energy solutions, such as the most recent EU Regulation on Batteries and Waste Batteries. According to the IEA analysis, companies specialising in Li development witnessed 50% rise in their investment spending. Fuelled by the rising demand of electric vehicles, large industrial groups are competing now in a quest to secure mineral supplies: General Motors announced a 650 million USD in Lithium Americas, while Tesla confirmed already plans to build a Li refinery in Texas (USA).
Along with its ‘Critical Minerals Market Review 2023’, the IEA also launched the IEA Critical Minerals Data Explorer, an interactive tool that facilitates access to the agency’s projection data.
The IEA analysis conclusions raise the concern of the diversity supply. The LiCORNE project was launched at the encounter of European aspirations to advance the energy transition. The project aims to increase the European Lithium (Li) processing and refining capacity to produce battery-grade chemicals from ores, brines and off-specification battery cathode materials. Over a span of 48 months, from the 1st of October 2022 to the 30th of September 2026, eight research and development centres in Europe will investigate no less than 14 new technologies for extracting, recovering and refining Li.
Currently in its first year, the LiCORNE project completed the task of characterising and providing materials for the R&D activities. Most of the materials are sourced from European resources, including spodumene and Li-rich mica from mines in France and Austria, and geothermal brine sampled from the Upper Rhine Graben (France and Germany). The synthetic brine is prepared in UK. Only continental brine and off-specification cathode material originate from non-European countries – Chile and Korea.
For more information, refer to the detailed article, and explore the available Li resources in Europe.
VITO achieves direct lithium extraction, using the Gas-Diffusion Electrocrystallisation (GDEx) technology. GDEx uses gas-diffusion electrodes to achieve this goal, by producing in-situ the necessary quantities of mild chemicals, which in turn form precipitates containing lithium.
During this period, the GDEx team has conducted experiments with synthetic solutions. The effect of adding chemical supplements to the process is being investigated to optimise the lithium recovery yield and selectivity vs. competing ions in solution. After optimising the GDEx process with synthetic streams and learning about the precipitating mechanisms, we are looking forward to extending the process in various geothermal brine solutions obtained from the consortium partners. After precipitation in the form of layered-double hydroxides, the GDEx team will investigate the downstream steps to obtain battery-grade lithium hydroxide.
More information about the GDEx process can be found at http://gdex.vito.be