
Reactor used for the solvometallurgical leaching experiments by TEC
Various research partners involved in the LiCORNE project have been exploring different Li extraction technologies from Li-rich ores, tailings and off-specification cathode materials from battery production. All these exploratory routes, including alkaline leaching [NTUA], advanced solvometallurgy [TEC] and reactive ball-milling [KIT], share common objectives, aiming to be more energy efficient and reduce the environmental impact.
TEC’s advanced solvometallurgy approach leverages deep eutectic solvents to extract lithium, providing an energy-efficient solution for selective removal. This technique is not only applicable to Li but also extends to the extraction of other critical elements contained in the off-spec cathode materials.
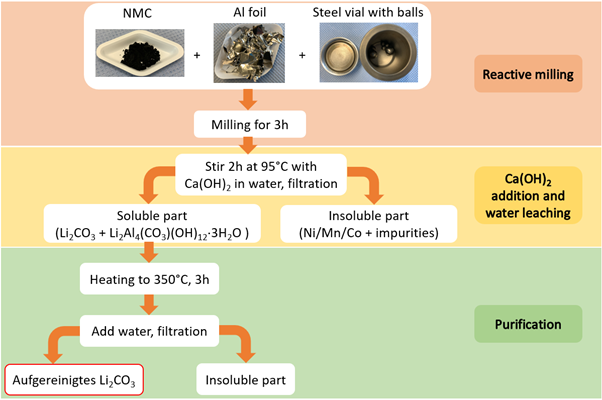
Meanwhile, KIT’s reactive ball-milling method is being explored as an effective battery recycling process. This innovative approach uses aluminium as a reducing agent for transition metals, which is already present in the input waste stream as the current collector material for electrodes. The process offers a direct route to battery-grade lithium carbonate
TEC investigated and developed a solvometallurgical extraction process for lithium from spodumene concentrate, lithic mica and lithium phosphate, and for lithium, cobalt and nickel from off-specification cathode material. The optimised operating conditions and necessary pre-treatment steps enabled over 95% extraction of Li, Co and Ni from these materials at room temperature. Additionally, the reuse of the organic solvents utilised during the leaching processes was effectively tested proving that it does not affect the yield in the next cycles. The lithium containing liquid streams obtained are processed by TEC in further steps with different technologies towards the obtention of pure battery-grade lithium carbonate.

Reactor used for the solvometallurgical leaching experiments by TEC
Researchers at KIT studied in depth various ball-milling parameters for the mechanochemical transformation of the off-specification cathode material samples provided by Umicore. Subsequent water leaching facilitated the separation of an insoluble metallic composite containing Ni, Mn and Co from water soluble Li-compounds. KIT researchers optimised various reducing agents – such as Al, Ca and Mg, achieving Li recovery exceeding 80 %, with a Li2CO3 purity of around 90 %.
Product streams obtained by the various extraction technologies here explored will be further processed in subsequent separation and purification processes and lithium recovery methods. © KIT
Product streams obtained by the various extraction technologies here explored will be further processed in subsequent separation and purification processes and lithium recovery methods.
During conventional mineral processing, significant resources are often lost during the beneficiation phase. Lithium-bearing particles trapped in the gangue can proceed to downstream stages, reducing the efficiency of the entire extraction process. To address this, researchers at TU Delft have developed an Opto-Magnetic Sorting System that significantly enhances the separation of lithium ores. This innovative technology combines precision liquid deposition and magnetic separation techniques, offering an advanced alternative to traditional gravity-based separation methods used in beneficiation circuits.
The process starts with lithium-bearing ores being crushed and sieved, isolating particles in the 2–4 mm size range for the next step – optical sorting. A high-resolution line scan camera captures continuous images of particles on a conveyor belt. These images are processed in real-time using a custom algorithm developed at TU Delft, which is trained to identify lithium-rich particles based on subtle colour differences.
Once identified, the target particles are selectively marked using magnetic powder. This enables the marked lithium-rich particles to be separated efficiently by a downstream magnetic conveyor into a dedicated container.
This innovative beneficiation approach has successfully prevented around 45% of the gangue material from entering the downstream process—nearly three times more efficient than the initially targeted improvement of 15%.
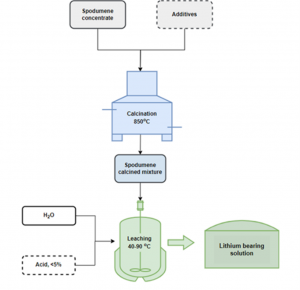
According to the State-of-the-Art [SoA], processing spodumene takes place at high-temperatures [1100oC], with direct implications on the economic viability of the entire process. Researchers at TEC have been investigating an alternative to conventional processes. Their investigation features ball milling and calcination at lower temperatures than the conventional process, using additives when needed for the improvement of the next leaching step.
Ball milling is a mechanical process that induces self-sustaining reactions in many sufficiently exothermic powder mixtures. These exothermic reactions, which release a significant amount of heat, can influence both the microscopic and macroscopic properties of the resulting material. On a microscopic level, the heat generated by the reactions can cause changes in the crystal structure and composition of the material. On a macroscopic level, these changes can affect the material’s overall properties, such as its strength, hardness and reactivity. TECNALIA’s findings show that the combination of the ball milling with additives lower calcination temperatures required [200oC below the SoA] in the pre-treatment process of the samples and, also, allow milder conditions in the next processing phases (leaching).
The process, replicated on lithic mica and lithium phosphate materials, were also successful to achieve good results in the next leaching step.

The furnace used in the calcination pre-treatment by TECNALIA
A large group of people arriving on 15 May to Milos island was gathering at Adamantas Conference Centre for the LiCORNE Dissemination Event. A homogenous group of participants, both local authorities and stakeholders from all conrners of Europe, some privileged and attending in person, others online, opened up the floor for discussions around innovative approaches for sustainable extraction of critical raw materials (CRMs) and the role of geothermal fields.
Organised by AdMIRIS on the Milos island, known for its rich geothermal and mineral resources, the event addressed mining sustainability and lithium market dynamics at international, European and national level.
Europe, from its position as an ambassador of the green transition, is expected to see a robust increase in demand for lithium. Although not as well endowed when it comes to lithium as Australia, China and Chile, it is still home to an estimated 5 % of the global reserves. Its insignificant contribution to the global supply highlights the need for strategic reserves and investments in mining and recycling to ensure a stable supply and resilience against market volatility. Presentations at LiCORNE’s Dissemination Event hinted at timely investments in strategic reserves of lithium while prices remain low. This would, ideally, run in parallel with investing in new mines in Europe, incentives for recycling initiatives and continuous development of a performant infrastructure to support the adoption of electric vehicles.
After a brief introduction into the strategic importance of lithium as a critical raw material for green technologies, Dr. Christos Kanellopoulos from the Hellenic Survey of Geology and Mineral Exploration (HSGME), mapped various Li deposits on the map of Greece, along with national exploration projects currently assessing the metal’s presence in various ore deposits – pegmatites, lignite deposits, high salinity closed lakes, geothermal fluids and tertiary basins.
Getting lithium out of European ground is not easy. The metal can be found mostly in hard-rock deposits, which require open pit mines that are usually large, polluting, water-intensive and noisy. Mining projects in Europe are often met with hostile attitudes by the “not-in-my-backyard” and environmental groups. The Greek perception on the mining context in Europe, presented by Mr. Konstantinos Yazitzoglou, Chairman of the Greek Mining Enterprises Association, was both awakening and engaging.
The first take-away set the context, which reminded clearly that all human activities, including mining, create an impact on the environment. Our challenge today is to balance this impact with the benefits it brings. Historically, Western countries have subcontracted mining activities to other parts of the world, often disregarding the environmental and social impacts. Today, this practice is no longer sustainable as those regions are no longer willing to bear the negative consequences.
The BRICS group controls a significant portion of the world’s critical raw materials. With this challenging scenario, Europe has initiated strategic projects and legislative measures to address this issue, but progress has been relatively slow.
The mining industry carries a few ‘negative images’ – including incidents, professional provocateurs and spontaneous reactions from local communities, that should be addressed if Europe aims to resuscitate its mining activities.
To foster a healthy relationship with local communities, finding common grounds on how to disagree and addressing concerns with full transparency remain essential. Emphasising the social dimensions of mining, Mr. Konstantinos Yazitzoglou presented a Greek initiative to create a network of mining township to promote the coexistence of mining and local communities.
During the second part, the LiCORNE Dissemination event spiced up with contradictory presentations. PPC Renewables, operating numerous wind farms, hydroelectric and photovoltaic plants and a hybrid power plant, has recently leased several geothermal concessions in different regions in Greece, including one in Milos-Kimolos-Polyaigos island group. Geophysical surveys and drilling have revealed significant geothermal potential in Milos. Key findings include high conductivity areas in the eastern part of the island, a clear division of the island into two geological sections, and the presence of geothermal fluids in the eastern part of the island.
Despite the richness of the geological formations and the company’s initiatives to engage with the local community, during the event, local authorities made an announcement that no geothermal explorations will take place on the Milos island.
However, the island already has exemplary use cases of mining activities nurtured through social responsibility and engagement with local communities. Imerys is a world leader in mineral-based specialties, providing high-added solutions to various industries, including construction and consumer goods. The company, also a partner in the LiCORNE project, succeeds through performant operations, commercial excellence, market-driven innovation and a strong business model.
In 2018, Imerys launched their SustainAgility programme, structured into three key areas:
Imerys use case reflecting their activities on the Milos island is a sustained effort across several years. Three to four years of corporate involvement in identifying stakeholders, analysing their influence and interests, managing relationships, planning and reporting outcomes through consultation, communication, negotiation, compromise and building relationships that stand the test of time. Imerys presence in Milos, especially during the Covid pandemic, ensured the island’s resilience in times when tourism regular activities were restricted. Imerys long-term operation in Milos relies on balanced development, co-existing with tourism businesses. The company has invested in various initiatives to secure social acceptance and support from the local community.
Various partners in the LiCORNE project presented their innovative research and innovation [R&I] approaches aimed at supporting the sustainable exploration and exploitation of lithium resources. These partners showcased cutting-edge technologies and methodologies designed to minimise environmental impact while maximising resource efficiency, ensuring that lithium extraction aligns with sustainability goals and contributes to the green energy transition.
The LiCORNE project coordinator, Dr. Lourdes Yurramendi opened the technical sessions with an introduction into the scope of work and the objectives, leading after the conversation to the presentations of the specific technologies explored by the LiCORNE partners:
At the LiCORNE EU Project Dissemination event, Nader Akil [Business Operations Manager at PNO] outlined how the EU’s funding is strategically distributed to support R&I initiatives like LiCORNE. The EU’s evolving policy mix, including the Critical Raw Materials Act [CRMA], proposed in March 2023, focuses on ensuring a diverse and secure supply of materials for new industries, setting priorities and benchmarks for 2030. The NetZero Industry Act [NZIA] aims to scale up clean technology manufacturing in the EU to 40 %, with fast-track permitting and strategic projects. The Innovation Fund, closely tied to the NZIA, supports net-zero technologies, including €1 billion for electric vehicle battery cell manufacturing and funding for lithium extraction combined with geothermal installations. The Competitiveness Compass aims to retain Europe’s competitive edge by closing the innovation gap and decarbonising high-impact sectors. With over €22.5 billion in strategic project investments and ambitious 2030 benchmarks, structured innovation ecosystems are essential.
In other research facilities, in different corners of Europe, other LiCORNE partners are reporting progress in producing battery-grade materials from various sources – brines, ores (spodumene for example) and off-specification cathode material.
Using the solutions derived from VITO-CAST team’s upstream processes, SINTEF researchers have constructed and commissioned electrochemical cells for electrodialysis of lithium chloride (LiCl) and lithium sulphate (Li2SO4) solutions. Researchers identified the optimal parameters to produce lithium hydroxide (LiOH) or lithium carbonate (Li2CO3), which achieved a current efficiency of approx. 40 % and a specific energy consumption of 20 kWh/kg. Further optimisation of the cell design is expected to reduce the energy consumption.

Membrane flow cell setup at SINTEF
Additionally, this process also produced a mix of Li2CO3 and LiOH through evaporative crystallisation, with a purity of almost 90 %, but showing sodium (Na) as the main impurity interfering with the process.
The organic-based membrane electrolysis, developed at TEC and tested on three types of solutions – those produced by the liquid-liquid extraction processes from brines and from spodumene leachates, and the off-specification cathode leachates – achieved up to 95 % Li yield, far beyond the levels established at the beginning of the project. Their tested carbonation method yielded a Li2CO3 with a purity exceeding 99% in the case of off-specification cathode material and spodumene concentrate materials. Not only the Li recovery target has been achieved, but also the solvent used in the former leaching process has been recovered and reused keeping the performance as initially, aiming for a more sustainable and circular process.

3-chamber Flow cell setup at TECNALIA
The research group at Fraunhofer Institute for Chemical Technology ICT explored a simple, highly scalable method for lithium purification using a combination of Ion Exchange (IE), Reversed Osmosis (RO) and Electrodialysis with bipolar membranes (EDBM) (see figure below). The goal was to recover high-purity lithium carbonate from Lithium-concentrated solutions provided by partners EnBW and KIT. The IE process effectively removed specific impurities (e.g. divalent cations). The significant level of impurities present in the solutions, provided by EnBW, prevented the electrodialysis with bipolar membranes. The EDBM process, applied uniquely on the samples sent by KIT, yielded a 99.89 % purity. However, the yield of the first precipitation step was determined to be around 35 %, highlighting the need for further optimisation in future precipitation cycles.

Setup to prepare Li2CO3 recovery from Li-concentrated solutions starting with ion exchange, via reversed osmosis and electrodialysis. © FRAUNHOFER
SINTEF researchers investigated the extraction of lithium and other valuable elements, such as Co, Ni, Mn from solid raw materials. They achieved selective chlorination of lithium from calcined spodumene concentrate and off-specification cathode waste in LiCl-KCl and CaCl2-NaCl-KCl melts. Theoretical assessments suggest that lithium can be subsequently electrowon from the LiCl-KCl mixture with a purity of approximately 99 %.

Chlorination apparatus at SINTEF
VITO-ELEC team focused on internally-developed Gas-Diffusion Electrocrystallisation (GDEx) technology, which demonstrated high efficiency – achieving lithium extraction rates more than 95 %. VITO-ELEC team successfully extracted lithium from various sources, including geothermal brines, effluents from sorption processing of hard rock beneficiation and the leachates of off-specification cathode materials.
The team has produced lithium carbonate from the extracted lithium by implementing a downstream synthesis procecure. The process achieved a >1 % lithium concentrate increase from geothermal brines and solid product eluates with over 20 % lithium concentration. Moreover, the energy consumption of the GDEx process was below 10 kWh per kg of Li2CO3, with over 90 % lithium recovery from all tested complex matrices.
According to its eponymous title, this article explains the purification technologies developed by LiCORNE partners to enhance lithium recovery from various sources –ore streams, geothermal and continental brines, mineral leachates and recycling streams. Researchers optimised each method to maximise lithium yield and selectivity, addressing key challenges such as interference from competing cations and material stability over multiple cycles.
Researchers at VITO developed a selective ion-exchange method using protonated titanium oxide [HTO] for the purification of lithium from spodumene streams. They shaped the lithium-titanium-oxide [LTO] adsorbents into spheres, which enabled dynamic testing. The spheres demonstrated separation factors larger than 100 for most metals studied, except for calcium (Ca2+), which is released during the regeneration step with lithium (Li+). After reporting 85 % Li recovery from aqueous alkaline spodumene leachates, VITO researchers recently achieved a selectivity of Li+ above 98 % after two cycles in batch mode. VITO is applying for a patent on a methodology which avoids the dissolution of titanium (Ti) during the acidic regeneration treatment, ensuring no Ti is dissolved in any of the 10 cycles tested.
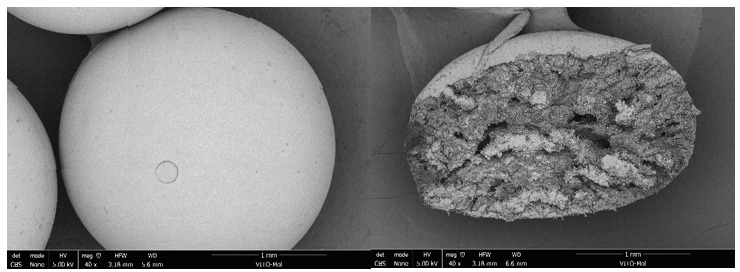
HTO based spheres utilized for lithium extraction from spodumene leachates. © VITO
TEC investigated ionic liquid-based extractants to recover lithium from geothermal and continental brines, as well as from leachates produced from mineral ores. These extractants offer an environmentally friendly alternative to the state-of-the-art methods that use harsh acidic conditions. The optimised operation conditions achieved high Li extraction yields (up to 95 %) with Li+ selectivity around 99 % for certain Li/cation combinations. This achievement surpasses results expected in the beginning of the project.
An additional benefit of this technology is its capacity to the reuse of the ionic liquids without compromising on the extraction performance.
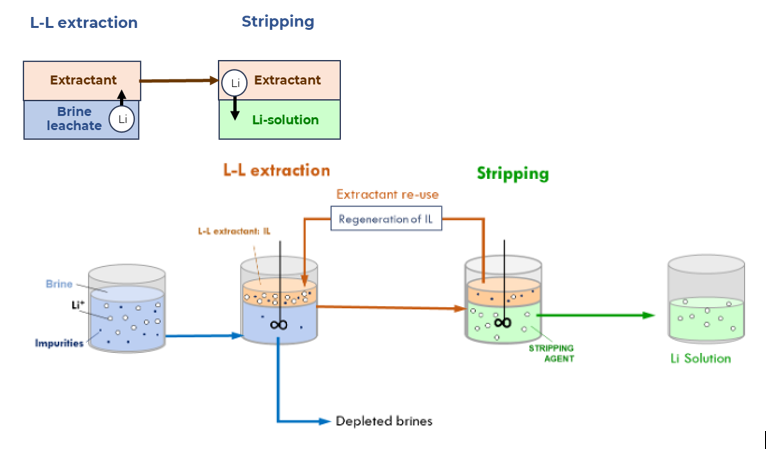
L-L extraction & stripping process by TECNALIA for the recovery of Li from brines and spodumene leachates. © TECNALIA
The research and development department at EnBW have been researching and developing a Li+ extraction technology to TRL4. Their goal is to develop a sustainable process to extract and separate Li+ from geothermal and continental brines, aiming at a minimum yield of 90 %.
copyright: EnBW
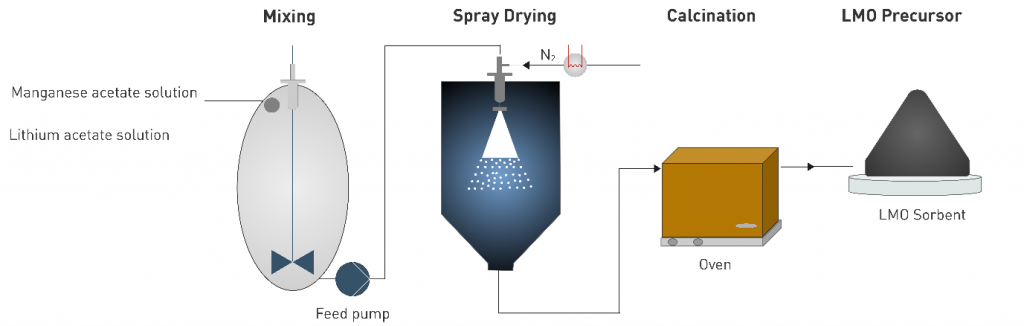
The optimisation of their technology features a novel synthesis route for Lithium Manganese Oxide [LMO] adsorbent. Doping with iron (Fe) or titanium (Ti) provided materials with better capacity and chemical stability. The recovery of lithium from geothermal brines was 92 % – a significant step beyond the state-of-the-art materials. EnBW researchers have recently submitted a patent application for the LMO technology, which shows good potential for future implementation at industrial level for Li recovery.
Research partners from the LiCORNE consortium are working on developing and optimising various technologies to produce battery-grade materials. SINTEF, for instance, have designed, built and tested their advanced electrodialysis apparatus using purified lithium (Li) solutions derived from the upstream treatment processes of Li-ore. Intermediary results show the process will require further optimisation to obtain 99 % purity LiOH and the targeted energy consumption of less than 15 kWh/kg. The research is still ongoing, focusing now on removing the Al ions prior to the electrodialysis process and on investigating new operating parameters.
In another task, working on the optimisation of the conditions for selective chlorination of spodumene concentrate and cathode waste, SINTEF achieved almost 95 % Li yield using CaCl2 -NaCl -KCl melts. Optimisation is underway to replicate the results to the other valuable materials available in the cathode material.
Researchers at TEC have been optimising the organic-based membrane electrolysis process to recover Li from organic solutions as Li2CO3. Results indicate they managed to achieve over 95 % Li yield from off-specification cathode material, while recovering all the organic solvent used in the previous (leaching) step for its reuse. Good yield rates have also been obtained for the treatment of solutions produced in the liquid/liquid [L/L] extraction of brines and spodumene. However, the selectivity of the membrane is insufficient to overcome the migration of the high concentration of other competing cations such as Na, K, Mg and Ca. Researchers are currently producing and testing new PIMs (Polymer Inclusion Membranes) to try to improve the results.
The research group at VITO have been refining their gas-diffusion electrocrystallisation process for Li recovery from brines, achieving over 95 % removal of Li from most of the samples. By manipulating and adding salts to the brine sample, results show that more than 99 % Li is extracted. The energy efficiency of the GDEx process can be improved with the optimisation of the GDEx reactor.
With all technological processes reporting progress and reaching the targets established at proposal stage, future months will rely on the results of the LCA and LCC analysis, which will establish the most promising processes that will enter the upscaling phase.
© visual:Adobe Stock Photos
Within the beneficiation process, the research group at TUD developed an opto-magnetically induced sorting technology. Within the next months, their work will continue developing their code to optimise the colour identification of target metals, and simultaneously on various set-ups to improve the magnetic attraction and and to ensure the seamless integration of all components of their opto-magnetically induced sorter.
Within the same work package, researchers at NTUA have developed a new calcination technology with additives, tested on spodumene concentrates. Using different settings and parameters, such as the processing temperature, reaction time, pressure, the extraction yields for Li ranged between 71 % and 96 %. Depending on the additive type, adjusting the calcination parameters accordingly can significantly reduce impurities, such as aluminum (Al), present in spodumene concentrate. Simultaneously, NTUA partners have been optimising a new technology for Li extraction with calcination from lithic mica and the results will be available in the upcoming communications.
Calcination scheme NTUA
Working on spodumene concentrates, the research group at TEC has established a novel pre-treatment process that allows a relevant improvement in the next leaching process of lithium for its valorisation. As a result of this method, which includes ball milling combined with additive, the transformation of the mineralogical structure of the spodumene takes place at a significantly reduced temperature, ranging from 1100ºC to 900ºC. Based on these findings, TEC has applied a similar approach for the lithium phosphate and the lithic mica materials, reporting good results.
© visual:Adobe Stock Photos
With no surprise, after Europe’s quest to replace fossil fuels and turn towards clean energy, lithium (Li) has been classified as a key component, making it to the short list of EU’s highly significant critical raw materials. With the transition to zero-emission vehicles, carmakers, as the most consuming industrial sector, will need ever more Li for batteries.
Renowned for its policy background, the EU decisional institutions adopted the Critical Raw Materials Act (CRMA) in record time. This accelerated adoption procedure shows nothing but the need for action, which reflects Europe’s urge to secure a sustainable supply of critical raw materials (CRMs). The CRMA sets specific targets to strengthen the EU’s capacities along the different stages of the value chain, ensuring that by 2030:
Both EnBW and LevertonHELM are key partners in the LiCORNE project. EnBW, as one of the largest energy supply companies in Germany and Europe, has the following tasks in the LiCORNE project: 1) to supply geothermal brine feedstock, respectively to conduct develop Li+ desorption technology aiming at min. 90% yield from geothermal and continental brines. LevertonHELM, on the other side, is a Lithium chemicals producer based in the UK, focusing on the manufacturing of a wide range of inorganic Li chemicals. In the framework of LiCORNE, the British company will benchmark and qualify the Li produced by the processes developed in the project, as battery-grade material.
German, respectively British companies have expanded their collaboration beyond the project’s framework, with a joint objective to advance the sustainable production of battery-grade Li carbonate and Li hydroxide – essential materials for electric mobility and energy storage solutions.
In previous articles, EnBW reported high Li concentrations for the geothermal brines in the Upper Rhine Valley (Bruchsal reservoir), ranging from 163 to 190 mg/L (Sanjuan et al., 2016). However, due to the characteristics of the reservoir, featuring highly mineralised brines, the extraction process was hampered by an elevated additional concentration of foreign ions (TDS 130 g/l). According to Laura Herrmann, Project Manager Research and Development at EnBW, the process requires increased selective adsorption technology in line with the exigences of the battery materials producers.
This industrial collaboration has resulted in a remarkable purity of 99.5% for lithium carbonate, demonstrating great potential for further scale-up to meet the EU’s demand for lithium.
Using direct Li extraction by adsorption (A-DLE), the process coordinated by the industrial partnership led to a remarkable purity of 99.5 % for Li carbonate. This successful initial trial holds promise for future upscale, potentially meeting the EU’s demand for Li.
Register to the Symposium on Direct Lithium Extraction
© visual:EnBW
Europe stands at a turning point in its journey towards establishing a competitive European value chain for batteries. Important steps have been taken in encouraging battery manufacturing plants, only to mention here the inauguration of the first gigafactory by Northvolt in Sweden. Yet, the market demand for batteries continues to surge, fueled not only by the electric vehicle sector but also by other mobility applications and stationary storage needs. The recently launched Quarterly EU Electricity Market Report Q3 ’23 indicates over 600,000 new battery electric vehicles (BEVs) were registered in Q3 ’23, 36% higher than the corresponding quarter in 2022 and counting for 24% market share.
In response to these record demands, the European batteries research and innovation (R&I) community has been dedicated to supporting the establishment of this industrial value chain in Europe, aided by public funding, including by the European Union. Various R&I projects under the umbrella of the BATT4EU Partnership (established under Horizon Europe Programme in 2021), LiCORNE included, are sharing forces within the Cluster Hub “Production of materials for batteries from European resources” to address common challenges.
Motivated by the global geopolitical developments, the strategic role batteries play in achieving Green Deal objectives and the ever-evolving nature of battery technologies, Europe recognises the critical need for strategic alignment among stakeholders. Replacing the BATT4EU SRIA of 2021 and the Batteries Europe SRA of 2020, the 2024 SRIA outlines key strategic actions that the European Batteries R&I Community will undertake to advance collaborative research projects facilitated by the BATT4EU Partnership. Different from the previous strategic agendas, the 2024 roadmap goes beyond specific chemistries, leveraging also the power of disruptive (digital) technologies to advance research across all battery types, including material science, manufacturing and recycling processes.
The new agenda draws on the roadmaps published by Batteries Europe and Battery 2030+, compiling inputs from numerous European battery experts, offering recommendations on short, medium, and long-term objectives. It emphasises the need for coordinated action not only at the European level but also within national and regional programmes.
The 2024 SRIA points to the following six imperatives which are necessary to set the foundations and support a competitive battery value chain in Europe:
• Ensure that (BATT4EU) research results reach gigafactories and the markets, through pilots, demonstrators and improved decision making aided by digital tools.
• Increase the strategic autonomy of Europe by reducing the reliance on foreign critical raw materials by supporting local and circular supply chains and support research into different battery chemistries, including sodium-ion technologies.
• Improve battery affordability to accelerate the green transition and keep the European industry competitive by improving batteries based on materials that are more abundant and pushing for better integration into end-use applications.
• Improve the flexibility of battery manufacturing and recycling systems to reduce lock-in effects and respond quickly to changes in a rapidly developing industry.
• Implement a safe and sustainable by design framework for batteries, which plays to European strengths, and which will help reduce emissions and use of substances of concern, improve safety and allow for the integration of smart functionalities.
• Support the continuity of excellent European battery research and academic-industrial cooperation by improving access to research facilities and pilot lines, use research projects to build up a skilled workface, and by avoiding gaps in research through continued funding, which will bind talented researchers to Europe.
BATT4EU Partnership is organising a webinar on 20 March 2024, between 10:00 and 11:30 Brussels time. The aim is to present the official document and to host engaging discussions with the experts behind this publication who will explain how this document will redefine the dynamics for the European battery sector.
Register here
On Thursday, 16 November, during the 2023 edition of the Raw Materials Week, the twelve EU funded projects that constitute the Cluster Hub ‘Materials for batteries’ gathered for their annual event in Brussels.
The Cluster Hub has been initiated last year during the 7th edition of the Raw Materials Week. The main objective of the meeting was to meet and discuss the latest developments in the participating projects as well as the new challenges and opportunities discovered through the projects’ lifetime. Nader Akil, Operations Manager at PNO Innovation, inaugurated this second edition outlining the motivation behind the hub’s establishment. He underlined the positive reception and sustained interest from various stakeholders keen on joining this initiative.
Discover and/or rediscover the first edition of the Cluster Hub workshop
Co-organised by RELiEF, EXCEED, ENICON and RAWMINA, the event was also the opportunity to welcome the four new members of the Cluster (EXCEED, RAWMINA, METALLICO and CRM-geothermal). the workshop gathered nearly 100 organisations driving the production and the recycling of raw materials for battery applications from primary and secondary resources.
Building on the initial objective of creating an environment that could foster knowledge exchange on different approaches for the recycling and recovery for battery applications, the event focused on three major topics that depict the transversality characterising the projects: the raw materials through research and science, the roles and challenges of industry and market for raw materials, and the raw materials under the scope of sustainability, durability and social acceptance. During this annual meeting, an interactive session led by Anish Patil from TechConcepts and representing the RELiEF project had the objective of Mapping the European battery material recycling landscape – more details to be found below, in the section referring to the interactive session.
The first session was moderated by Sonia Matencio from LEITAT, representing the RAWMINA project. This session had the objective of discussing the raw materials through research and science, under the scopes of mining, refining, processing as well as the battery data. Sonia introduced this topic under the scope of RAWMINA, explaining the integrated innovative pilot system for Critical Raw Materials recovery from mine waste in a circular economy context. To this end, Christophe Aucher, from LEITAT as well, highlighted the need on an open battery passport system to better reflect and account for any adaptations that might be required due to the changing regulatory landscape.
Sonia welcomed afterward Brecht Dewulf from KU LEUVEN and representing ENICON, who discussed the sustainable processing of Europe’s low grade sulphidic and lateritic Ni/Co ores and tailings into battery grade metals. The idea behind this was to show all the potential of Ni/Co resources for Europe.
Xochitl Dominguez from VITO concentrated her speech on gas-diffusion electrocrytallisation (GDEx), a crucial topic for the projects LiCORNE and RHINOCEROS she works with. GDEx is an electrochemical process of reactive precipitation of metals in solution with oxidising or reducing agents produced in-situ by the electrochemical reduction of a gas, in a gas-diffusion electrode. This was followed by Katrin Kieling from GFZ Potsdam, working there for the CRM-geothermal project and shortly explained the challenges of extracting critical raw materials from geothermal fluids. To conclude this first session, Sandra Pavón from Fraunhofer IKTS explained the demonstration of battery metals recovery from primary and secondary resources through a sustainable processing methodology in the METALLICO project.
Discover presentations from Session 1
The annual meeting followed its course with an interactive session led by Anish Patil, which scrutinised stakeholders’ perspectives on the Green Deal Industrial Plan, Net Zero Industrial Act, Critical Raw Materials Act and the European Battery Regulation 2023. Mentimeter facilitated this interactive session, engaging the audience to explore how these policies intersect, complement each other, and identify critical measures and incentives for achieving their objectives.
Over 30 persons participated in the live-poll proposed, which results display the priority to be set on funding and state aid regarding ranking the four pillars of the Green Deal Industrial Plan in order of relevance (followed by skills development, conductive regulation, and open and fair trade). Another major topic regarding the stimulation of investment in net Zero technologies, the majority of answers placed the ‘enhanced skills’ as first priority, shortly followed by facilitating the access to the market.
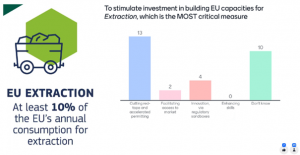
Lastly, the participants were divided regarding the critical measures to implement in the EU to stimulate investment in building domestic capacities for extraction of critical raw materials (CRMs). Although the majority opted for ‘cutting red-tape and accelerated permitting’, approximately half of the answers evoked uncertainty, which emphasised one more time the need to engage with policy makers as external stakeholders in all projects.
This interactive workshop was followed by two sessions, which aimed at discussing the challenges and opportunities of raw materials within the frame of industry and market, as well as the social acceptance, sustainability, and durability.
Alan Gonzalez from PNO Innovation Begium, representing LiCORNE, moderated the industry part, whereas Sam Hoefman from RELiEF moderated the last session on social acceptance, sustainability, and durability. Distinguished panellists took the stage to engage in debates on various topics.
Edvarts Emerson, Production and Testing Engineer at Watt4Ever, presented his work on the benchmark depository of 2nd life use of lithium in batteries, acceptance criteria and guidelines, work developed within the RHINOCEROS project. Benjamin Wilson, representing the RESPECT Project, displayed Aalto University’s work advancing efficient, sustainable, innovative and safe battery recycling processes in the EU. Laura Kainiemi from LUT University, representing the RELiEF Project, Konstantinos Komnitsas from the Technical University of Crete (TUC), on behalf of EXCEED, and Vitor Correia from INTRAW for the CRM-geothermal project, collectively debated the role and impact of social acceptance among affected communities, the importance of triggering new dialogues on responsible mining activities, and the joint involvement of regional, national and European authorities, academia, industry partners, and citizens in shaping these initiatives.
A big thank you to all participants for this co-creative and very constructive and inspiring meeting.